Fibroblast Growth Factor Receptor (FGFR) Signaling Pathway in Cancer and Inflammation
A topical collection in Cells (ISSN 2073-4409). This collection belongs to the section "Cell Signaling".
Viewed by 69622Editors
Interests: molecular determinants of malignant phenotype; network of cell signaling; endocytosis and intracellular sorting; nuclear transport; nuclear signaling; targeted anticancer therapy
Interests: protein engineering; FGF-signaling network; protein-protein interactions; intracellular transport; apoptosis; FGF-conjugates
Topical Collection Information
Dear Colleagues,
In the era of personalized medicine, tailored drugs targeting the underlying disease mechanisms or specific disease markers are of great importance.
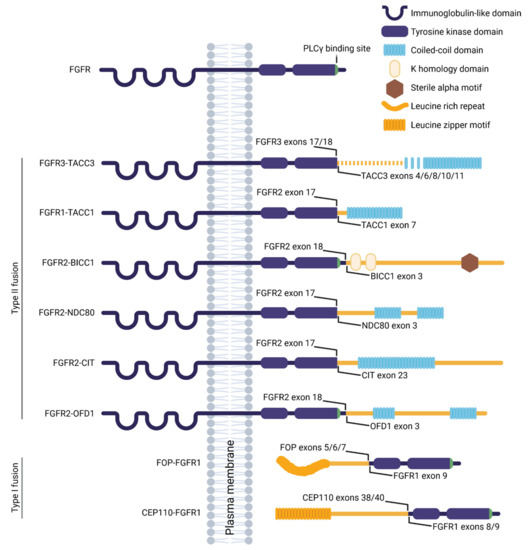
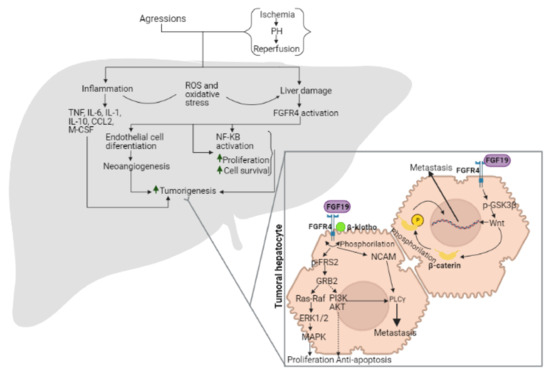
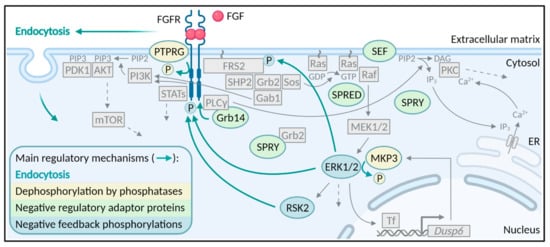
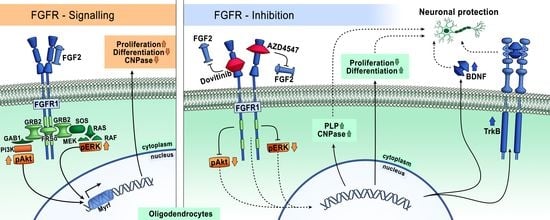
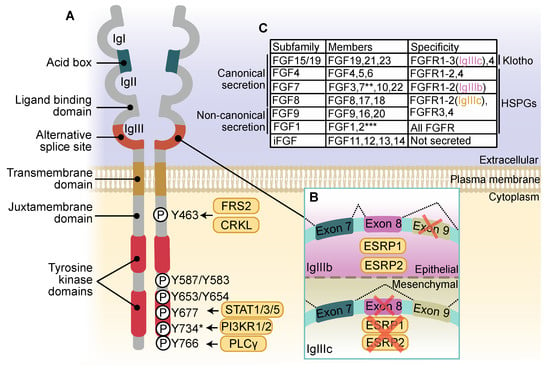
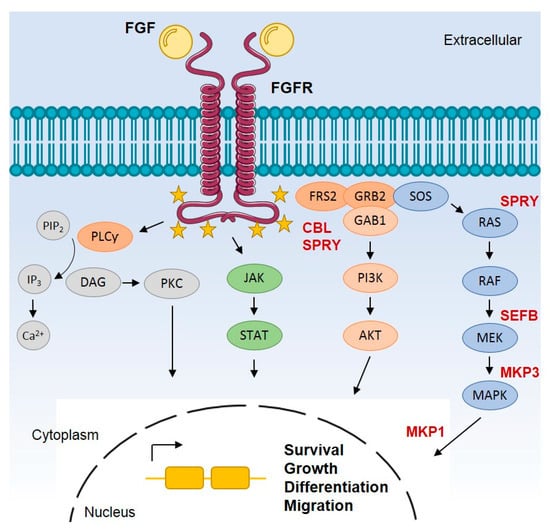
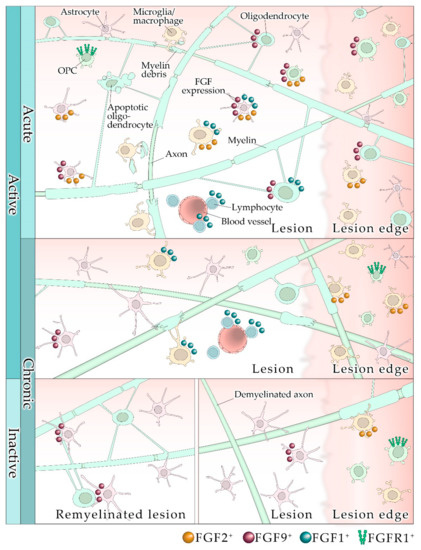
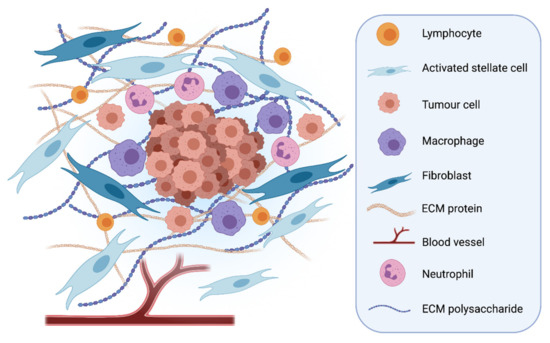
Fibroblast growth factor receptors (FGFRs) regulate a variety of cellular functions, from embryogenesis via organogenesis to adult tissue homeostasis. FGFR signaling also plays significant roles in the inflammation as well as in proliferation, invasion, and survival of several types of malignant diseases. FGFR-induced alterations, including gene amplification, chromosomal translocation, and mutations, have been shown to be associated with the tumor initiation and progression of many cancers. FGFR signaling has been extensively studied, and preclinical evidence indicates that activation of FGFRs is often common in the development of malignant tissue.
This Topical Collection aims to provide an overview of the current knowledge on the role of FGFR signaling in generation of an inflammatory response and in tumorigenesis, acquiring a malignant phenotype, metastasis process, and chemoresistance. It also summarizes all experience accumulated up to today indicating that FGFRs might be one of the most important molecular therapeutic targets in anticancer therapy.
We look forward to your contributions.
Dr. Antoni Wiedlocha
Dr. Malgorzata Zakrzewska
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- FGFRs
- signaling
- inflammation
- tumorigenesis
- malignancy
- therapeutic target