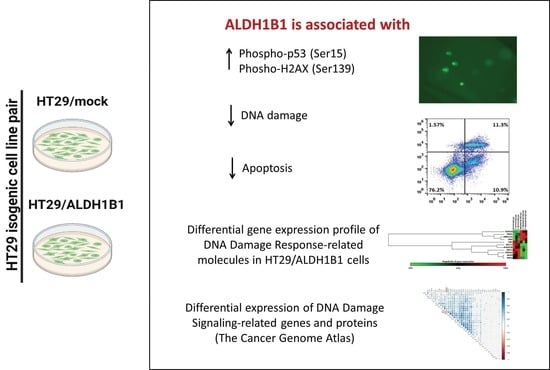
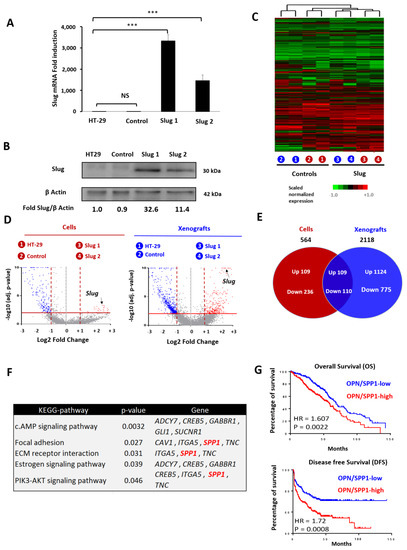
Molecular and Cellular Mechanisms of Cancers: Colorectal Cancer
A topical collection in Cells (ISSN 2073-4409). This collection belongs to the section "Cellular Pathology".
Viewed by 6689Editors
Interests: colorectal cancer; tumor-immmune interactions; cancer genetics and epigenetics; immunotherapy; biomarkers
Special Issues, Collections and Topics in MDPI journals
Interests: colorectal cancer; colorectal carcinogenesis; inflammation and metabolic risk factors for cancer; hereditary colorectal cancer; colorectal adenomas; aberrant crypt foci
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
With over 700,000 deaths world-wide annually, colorectal cancer remains a large societal burden. Our knowledge of the underlying biology of colorectal tumor formation is impressive, but still, we are not able to efficiently fight this disease. Recent insights are that we should not only focus on biological processes that take place in the tumor cell itself, steered by both genetic and epigenetic events, but also on the response of the environment. This Topic Collection aims to focus on that environmental interaction and its steering processes, and on the mechanisms involved in the early and later steps of colorectal cancer development. Finally, we will discuss the conditions that may lead to a better clinical approach to colorectal cancer.
We very much look forward to your contributions.
Dr. Peter Kuppen
Prof. Luca Roncucci
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- colorectal cancer
- tumor microenvironment
- tumor host response
- tumor genetics and epigenetics
- colorectal carcinogenesis
- colorectal cancer prevention