Molecular Mechanisms to Target Cellular Senescence in Aging and Disease
A topical collection in Cells (ISSN 2073-4409). This collection belongs to the section "Cellular Aging".
Viewed by 28699Editors
Interests: heart failure; adult stem cells; cell senescence; mechanotransduction; extracellular vesicles
Special Issues, Collections and Topics in MDPI journals
Interests: biology of aging; biogerontology; experimenetal gerontology; geroscience; cellular senescence
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
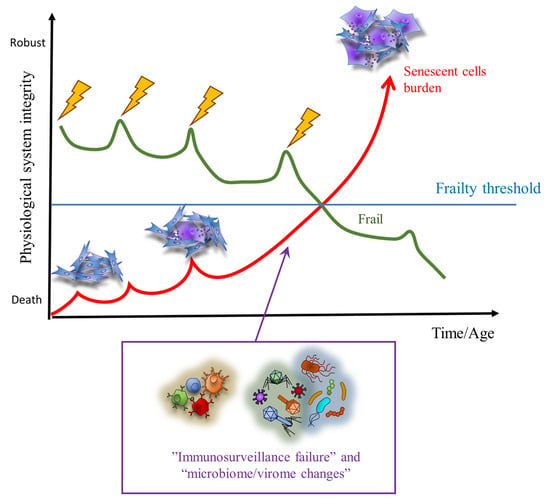
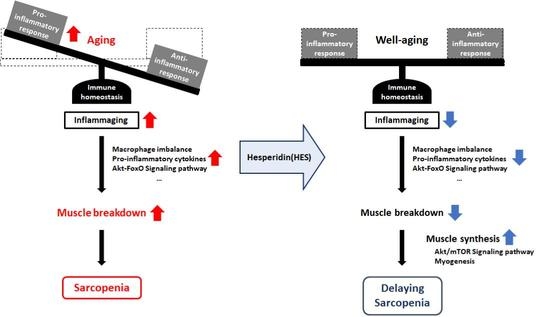
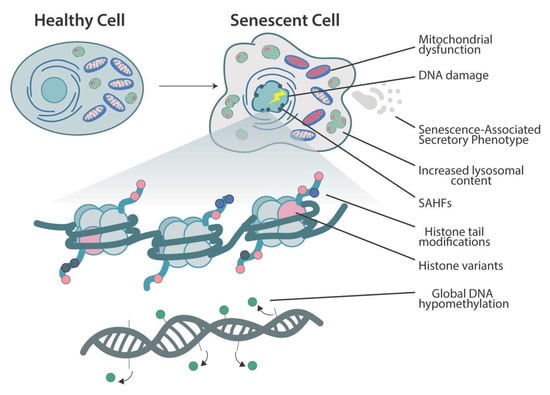
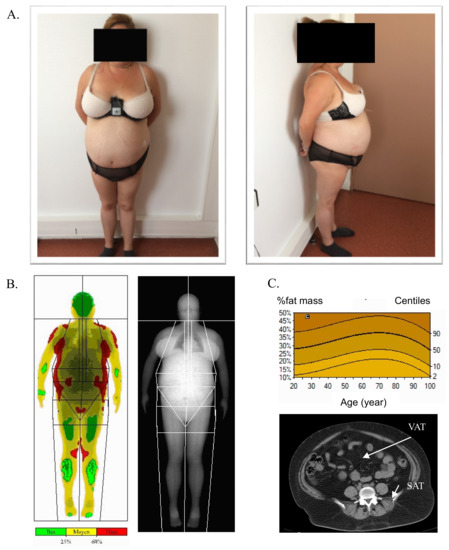
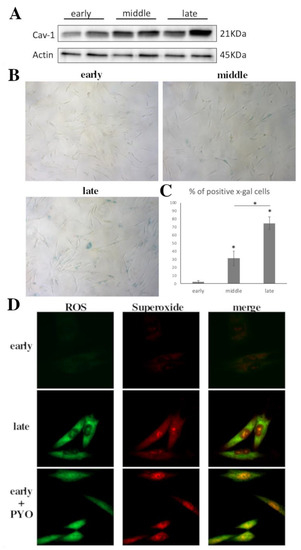
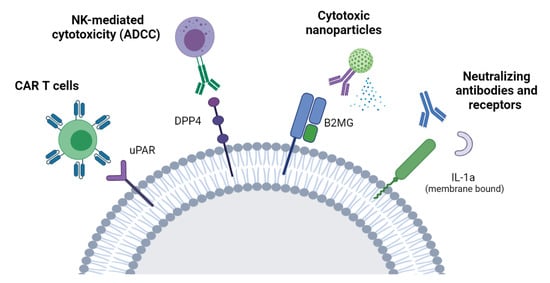
The term cellular senescence indicates a complex cellular response to a variety of stressors that results in the permanent withdrawal of potentially damaged cells from the cell cycle. Further, cellular senescence is associated with a modification of the secretome, promoting extracellular matrix remodeling, recruitment of inflammatory cells, angiogenesis, cell de-differentiation, and induction of cellular senescence in a paracrine fashion. It is conceivable that cellular senescence has been positively selected by evolution both as a barrier to avoid cancerogenesis and to promote tissue repair. However, with aging, senescent cells progressively accumulate in tissues, thus reducing the organism’s capacity to replace lost cells either with normal wear and tear processes or following damage. The immune system plays a special role in these phenomena, as changes in the immune system associated with age may contribute to reduce senescence immunesurveillance (the elimination of senescent cells mediated by the immune system), thus promoting the accumulation of senescent cells in tissues. As a result, the organism becomes more and more vulnerable to minor injuries, while chronic, age-related pathologies develop. Moreover, the higher mortality rate of older people during the current COVID-19 pandemic has raised interest on how senescence impacts our host defense to respiratory viruses. A causal role played by accumulating senescent cells in the pathophysiology of most age-related pathologies is supported by a growing body of literature. Indeed, genetic and drug-based interventions have been tested, aiming at eradicating senescent cells from old tissues with the intent to prevent chronic pathologies and delay aging.
This Topical Collection aims to collect a series of original research and review articles addressing the exciting and emerging field of cellular senescence in aging and age-related pathologies. We would like to put the emphasis on the molecular mechanism that is responsible for the phenomenon and that could be targeted both for diagnostics and therapeutic purposes. Suggested potential topics may be: stem cell senescence in age-related pathologies; mechanisms of proteostasis failure in chronic pathologies; mechanisms of immune surveillance failure in aging and disease; senolytic and rejuvenating therapies; exosomes as therapeutic or diagnostic tools; cell senescence and cancer; cell senescence and infectious diseases, as well as new models and therapeutic tools to target senescence in aging.
Prof. Antonio Paolo Beltrami
Dr. Marco Malavolta
Guest Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- cellular senescence
- proteostasis
- inflammation
- inflammaging
- immunosenescence
- stem cells
- exosomes
- extracellular vesicles
- senolytics
- aging