Cellular and Molecular Mechanisms of Atherosclerosis
A topical collection in Cells (ISSN 2073-4409). This collection belongs to the section "Cells of the Cardiovascular System".
Viewed by 28447Editors
Interests: ahterosclerosis; inflammation; NF-kB; nulcear receptor; endocrine disrupting chemicals
Topical Collection Information
Dear Colleagues,
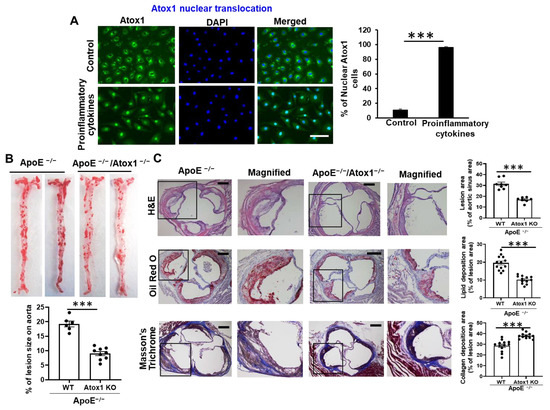
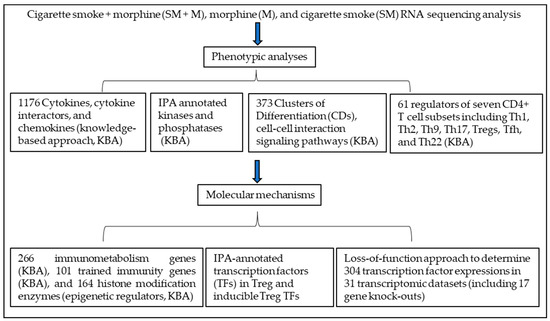
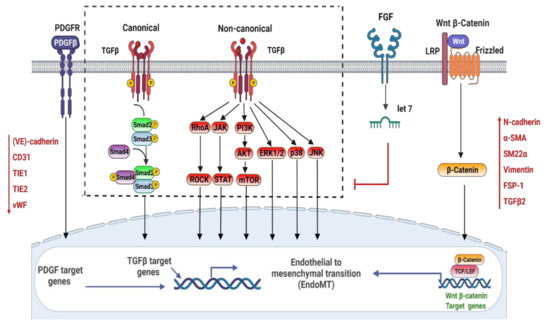
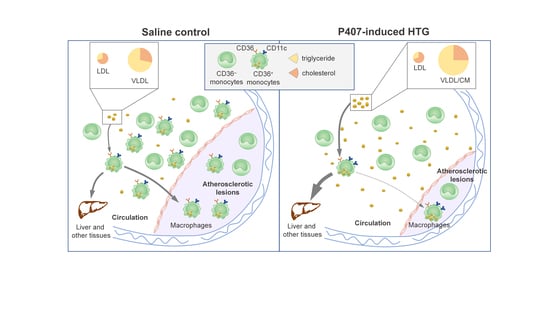
Atherosclerotic cardiovascular disease (CVD) is still the leading cause of mortality and morbidity worldwide despite advanced diagnosis and treatments. Atherosclerosis is a chronic inflammatory disease characterized by the accumulation of lipids and inflammatory molecules in the subendothelium of the artery, leading to restricted blood flow and ultimately myocardial infarction and stroke. Atherosclerosis is a complex chronic disease involving the interaction of genetic and environmental factors over multiple years. A multitude of treatments have been developed to halt or reverse the progression of this disease, including lifestyle changes, interventional procedures such as angioplasty and stent placement, and the prescription of medications to lower blood cholesterol levels, prevent clot formation, and reduce blood pressure. While these treatments have undoubtedly improved outcomes, there is more room for improvement. As a result, new classes of therapeutics are being developed to address the shortcomings of existing treatments. At the same time, inquiries into the basic biological mechanisms of atherosclerosis continue at a rapid pace.
In this Special Issue of Cells, we invite you to contribute original research articles, reviews, or shorter perspective articles on all aspects related to the theme of “Atherosclerosis”. Expert articles describing the mechanistic, functional, cellular, biochemical, or general aspects of atherosclerosis are highly welcome.
Dr. Changcheng Zhou
Dr. Hong Chen
Guest Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- signaling pathways
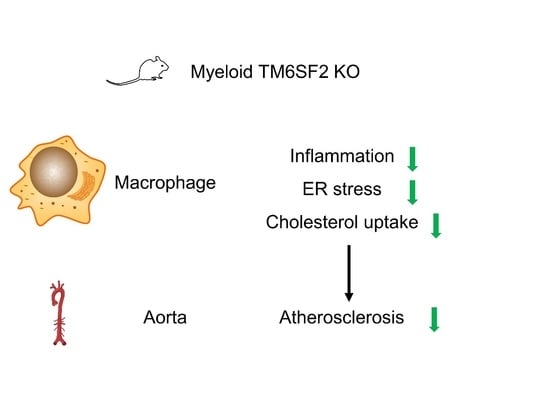
- macrophage function
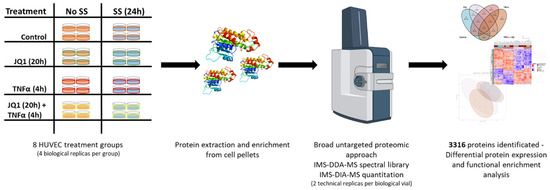
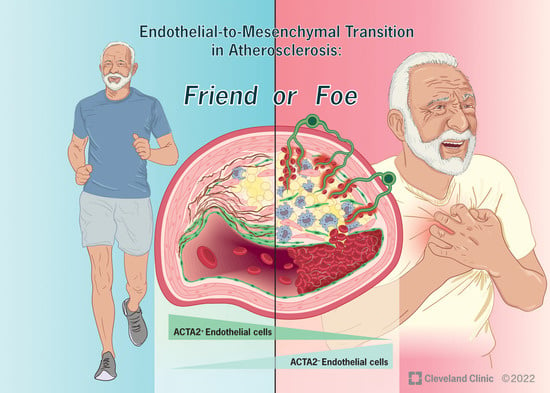
- endothelial cell function and endothelial–mesenchymal transition in atherosclerosis
- smooth muscle cell function and smooth muscle transdifferentiation in atherosclerosis
- lipid homeostasis
- mechanotransduction
- system biology
- environmental exposure
- novel biomarkers
- microbiota metabolism
- in vitro and in vivo models
- atherosclerosis calcification
- small noncoding rnas and long noncoding rnas
- covid-19
- bioimaging
- translational medicine
- sex differences in atherosclerosis
- new therapeutics