Feature Papers in Cell Motility and Adhesion
Share This Topical Collection
Editor
 Dr. Francisco Rivero
Dr. Francisco Rivero
 Dr. Francisco Rivero
Dr. Francisco Rivero
E-Mail
Website
Collection Editor
Reader in Biomedical Science, Hull York Medical School, University of Hull, Hull HU6 7RX, UK
Interests: actin cytoskeleton; actin-binding proteins; Rho GTPases; cyclase-associated protein; coronin; plastin; cell motility; platelet biology; endothelial cell biology
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
This Topical Collection, “Feature Papers in Cell Motility and Adhesion”, aims to collect high-quality research articles, review articles, and communications on all aspects of cell motility and adhesion, with a focus on cell biology research. Since the goal of this Topical Collection is to illustrate, through selected works, frontier research in cell motility and adhesion, I encourage Editorial Board Members of Cells to contribute papers reflecting the latest progress in their research field or to invite relevant experts and colleagues to do so. We also welcome senior experts in this field to make contributions to this Topical Collection. Please kindly note that all invited papers will be published online free of charge once accepted.
Topics include, without being limited to, the following:
- Cytoskeleton—microtubules, microfilaments, and intermediate filaments;
- Membrane trafficking, cellular dynamics and interactions of integrins, cell adhesion molecules and ECM components;
- Functions and molecular interactions of actin-binding proteins, microtubule-binding proteins, and intermediate-filament-binding proteins;
- Molecular and cellular architecture of cell adhesion complexes and cell junctions;
- Cell division;
- Signaling processes in cytoskeleton remodeling, cell motility, and adhesion;
- Cell migration during embryonic and postnatal development;
- Cancer cell migration, invasion, and metastasis;
- Neurite outgrowth and axonal guidance;
- Immune synapse formation and function, immune cell migration and extravasation;
- Imaging technologies, novel biomaterials, and other instrumental/computational advances in studying cell motility and adhesion.
Dr. Francisco Rivero
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs).
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Published Papers (5 papers)
Open AccessArticle
Apoptotic Cell-Derived CD14(+) Microparticles Promote the Phagocytic Activity of Neutrophilic Precursor Cells in the Phagocytosis of Apoptotic Cells
by
Yu-Chieh Lin, Wen-Hui Tsai, Shao-Chi Chang and Hui-Chi Hsu
Viewed by 850
Abstract
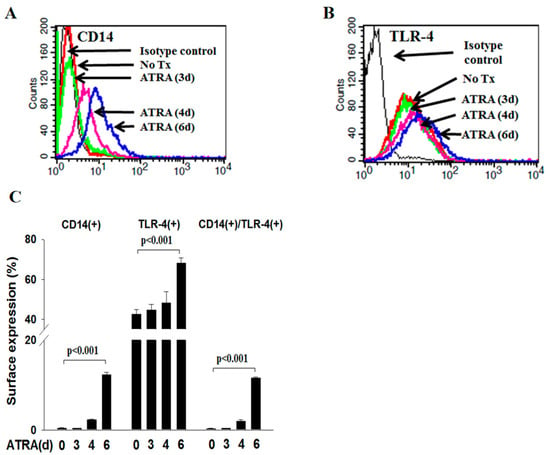
Membranous CD14 is crucial in the phagocytic activity of neutrophils. However, the role of CD14(+) microparticles (MPs) derived from apoptotic neutrophils (apo-MP) during the phagocytic process is not clear. All trans-retinoic acid (ATRA) induces acute promyelocytic leukemic NB4 cells along granulocytic differentiation. In
[...] Read more.
Membranous CD14 is crucial in the phagocytic activity of neutrophils. However, the role of CD14(+) microparticles (MPs) derived from apoptotic neutrophils (apo-MP) during the phagocytic process is not clear. All trans-retinoic acid (ATRA) induces acute promyelocytic leukemic NB4 cells along granulocytic differentiation. In this study, we investigated the role of CD14(+)apo-MP in the cell–cell interaction during the phagocytic process of apoptotic cells by viable ATRA-NB4 cells. We firstly demonstrate that CD14 expression and phagocytic activity of NB4 cells were upregulated simultaneously after ATRA treatment in a time-dependent manner, and both were significantly enhanced via concurrent lipopolysaccharide treatment. The phagocytic activity of ATRA-NB4 cells and lipopolysaccharide-treated ATRA-NB4 cells were both significantly attenuated by pre-treating cells with an antibody specific to either CD14 or TLR4. Further flow cytometric analysis demonstrates that apoptotic ATRA-NB4 cells release CD14(+)apo-MP in an idarubicin dosage-dependent manner. Both CD14 expression and the phagocytic activity of viable ATRA-NB4 cells were significantly enhanced after incubation with apo-MP harvested from apoptotic ATRA-NB4 cells, and the apo-MP-enhanced phagocytic activity was significantly attenuated by pre-treating apo-MP with an anti-CD14 antibody before incubation with viable cells. We conclude that CD14(+)apo-MP derived from apoptotic ATRA-NB4 cells promotes the phagocytic activity of viable ATRA-NB4 cells in engulfing apoptotic cells.
Full article
►▼
Show Figures
Open AccessArticle
YAP Activation in Promoting Negative Durotaxis and Acral Melanoma Progression
by
Yuxing Huang, Jing Su, Jiayong Liu, Xin Yi, Fang Zhou, Jiaran Zhang, Jiaxiang Wang, Xuan Meng, Lu Si and Congying Wu
Cited by 4 | Viewed by 1962
Abstract
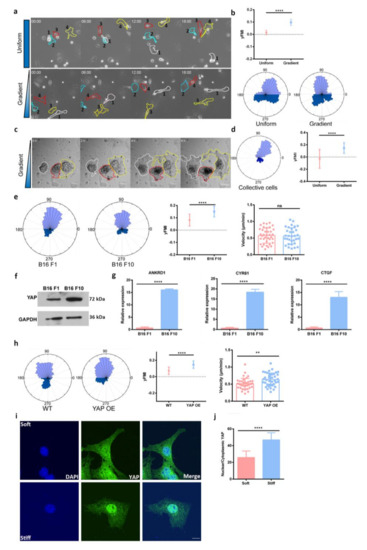
Directed cell migration towards a softer environment is called negative durotaxis. The mechanism and pathological relevance of negative durotaxis in tumor progression still requires in-depth investigation. Here, we report that YAP promotes the negative durotaxis of melanoma. We uncovered that the RhoA-myosin II
[...] Read more.
Directed cell migration towards a softer environment is called negative durotaxis. The mechanism and pathological relevance of negative durotaxis in tumor progression still requires in-depth investigation. Here, we report that YAP promotes the negative durotaxis of melanoma. We uncovered that the RhoA-myosin II pathway may underlie the YAP enhanced negative durotaxis of melanoma cells. Acral melanoma is the most common subtype of melanoma in non-Caucasians and tends to develop in a stress-bearing area. We report that acral melanoma patients exhibit YAP amplification and increased YAP activity. We detected a decreasing stiffness gradient from the tumor to the surrounding area in the acral melanoma microenvironment. We further identified that this stiffness gradient could facilitate the negative durotaxis of melanoma cells. Our study advanced the understanding of mechanical force and YAP in acral melanoma and we proposed negative durotaxis as a new mechanism for melanoma dissemination.
Full article
►▼
Show Figures
Open AccessEditor’s ChoiceArticle
Pharmacological Activation of Potassium Channel Kv11.1 with NS1643 Attenuates Triple Negative Breast Cancer Cell Migration by Promoting the Dephosphorylation of Caveolin-1
by
Ying Jiang, Vitalyi Senyuk, Ke Ma, Hui Chen, Xiang Qin, Shun Li, Yiyao Liu, Saverio Gentile and Richard D. Minshall
Cited by 5 | Viewed by 2206
Abstract
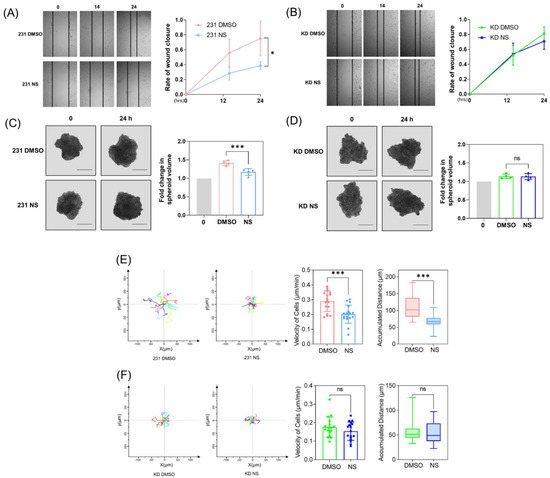
The prevention of metastasis is a central goal of cancer therapy. Caveolin-1 (Cav-1) is a structural membrane and scaffolding protein shown to be a key regulator of late-stage breast cancer metastasis. However, therapeutic strategies targeting Cav-1 are still lacking. Here, we demonstrate that
[...] Read more.
The prevention of metastasis is a central goal of cancer therapy. Caveolin-1 (Cav-1) is a structural membrane and scaffolding protein shown to be a key regulator of late-stage breast cancer metastasis. However, therapeutic strategies targeting Cav-1 are still lacking. Here, we demonstrate that the pharmacological activation of potassium channel Kv11.1, which is uniquely expressed in MDA-MB-231 triple negative breast cancer cells (TNBCs) but not in normal MCF-10A cells, induces the dephosphorylation of Cav-1 Tyr-14 by promoting the Ca
2+-dependent stimulation of protein tyrosine phosphatase 1B (PTP1B). Consequently, the dephosphorylation of Cav-1 resulted in its disassociation from β-catenin, which enabled the accumulation of β-catenin at cell borders, where it facilitated the formation of cell–cell adhesion complexes via interactions with R-cadherin and desmosomal proteins. Kv11.1 activation-dependent Cav-1 dephosphorylation induced with NS1643 also reduced cell migration and invasion, consistent with its ability to regulate focal adhesion dynamics. Thus, this study sheds light on a novel pharmacological mechanism of promoting Cav-1 dephosphorylation, which may prove to be effective at reducing metastasis and promoting contact inhibition.
Full article
►▼
Show Figures
Open AccessArticle
Phosphoinositide 3 Kinase γ Plays a Critical Role in Acute Kidney Injury
by
Xiaogao Jin, Qinjun Chu, Liwei Sun, Melanie Tran and Yanlin Wang
Cited by 3 | Viewed by 1650
Abstract
Inflammatory cells contribute to the pathogenesis of renal ischemia-reperfusion injury (IRI). However, the signaling mechanisms underlying the infiltration of inflammatory cells into the kidney are not well understood. In this study, we examined the effects of phosphoinositide 3 kinase γ (PI3Kγ) on inflammatory
[...] Read more.
Inflammatory cells contribute to the pathogenesis of renal ischemia-reperfusion injury (IRI). However, the signaling mechanisms underlying the infiltration of inflammatory cells into the kidney are not well understood. In this study, we examined the effects of phosphoinositide 3 kinase γ (PI3Kγ) on inflammatory cells infiltration into the kidney in response to ischemia-reperfusion injury. Compared with wild-type mice, PI3Kγ knockout mice displayed less IRI in the kidney with fewer tubular apoptotic cell. Furthermore, PI3Kγ deficiency decreased the number of infiltrated neutrophils, macrophages, and T cells in the kidney, which was accompanied by a decrease in the expression of pro-inflammatory cytokines in the kidney. Moreover, wild-type mice treated with AS-605240, a selective PI3Kγ inhibitor, displayed less tubular damage, accumulated fewer inflammatory cells, and expressed less proinflammatory molecules in the kidney following IRI. These results demonstrate that PI3Kγ has a critical role in the pathogenesis of kidney damage in IRI, indicating that PI3Kγ inhibition may serve as a potential therapeutic strategy for the prevention of ischemia-reperfusion-induced kidney injury.
Full article
►▼
Show Figures
Open AccessArticle
Dendritic Cell Migration Is Tuned by Mechanical Stiffness of the Confining Space
by
Yongjun Choi, Jae-Eun Kwon and Yoon-Kyoung Cho
Cited by 14 | Viewed by 4410
Abstract
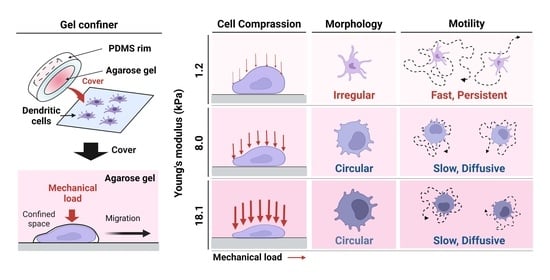
The coordination of cell migration of immune cells is a critical aspect of the immune response to pathogens. Dendritic cells (DCs), the sentinels of the immune system, are exposed to complex tissue microenvironments with a wide range of stiffnesses. Recent studies have revealed
[...] Read more.
The coordination of cell migration of immune cells is a critical aspect of the immune response to pathogens. Dendritic cells (DCs), the sentinels of the immune system, are exposed to complex tissue microenvironments with a wide range of stiffnesses. Recent studies have revealed the importance of mechanical cues in immune cell trafficking in confined 3D environments. However, the mechanism by which stiffness modulates the intrinsic motility of immature DCs remains poorly understood. Here, immature DCs were found to navigate confined spaces in a rapid and persistent manner, surveying a wide range when covered with compliant gels mimicking soft tissues. However, the speed and persistence time of random motility were both decreased by confinement in gels with higher stiffness, mimicking skin or diseased, fibrotic tissue. The impact of stiffness of surrounding tissue is crucial because most in vitro studies to date have been based on cellular locomotion when confined by microfabricated polydimethylsiloxane structures. Our study provides evidence for a role for environmental mechanical stiffness in the surveillance strategy of immature DCs in tissues.
Full article
►▼
Show Figures