Cell Death Mechanisms and Therapeutic Opportunities in Glioblastoma
A special issue of Cells (ISSN 2073-4409). This special issue belongs to the section "Cells of the Nervous System".
Deadline for manuscript submissions: closed (15 April 2024) | Viewed by 24828
Special Issue Editor
Special Issue Information
Dear Colleagues,
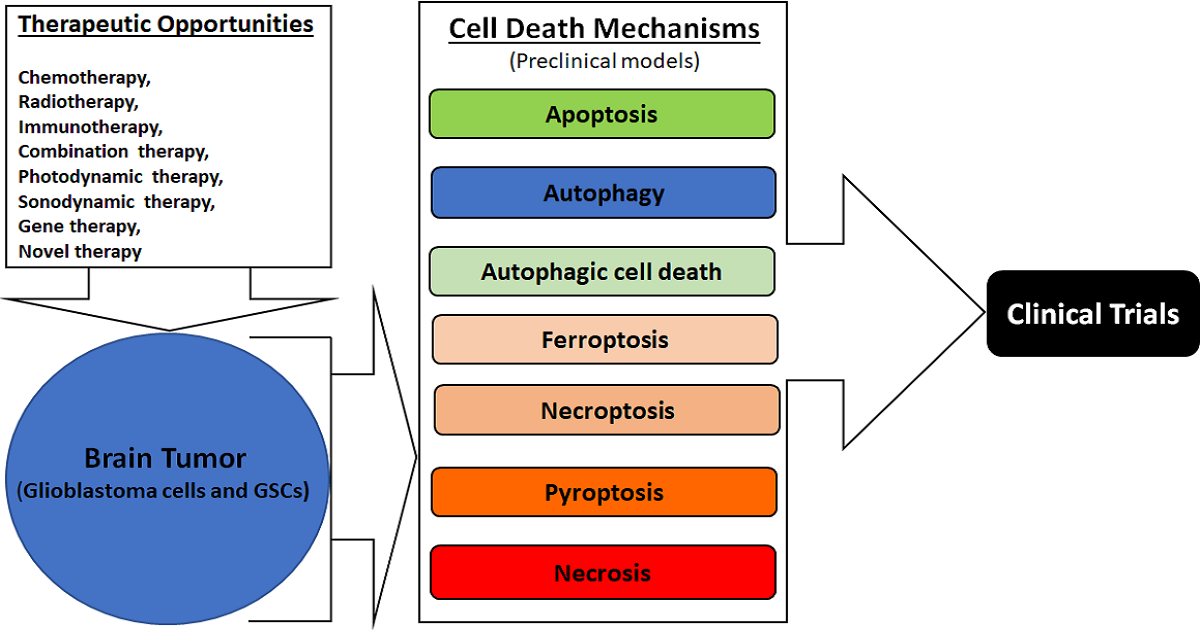
Cell death mechanisms, either induction or inhibition by specific therapeutic strategies, provide great opportunities for controlling the growth of glioblastoma multiforme, often simply called glioblastoma, the most malignant brain tumor in humans. Although there is an ever-increasing list of cell death mechanisms, about a half dozen of them (apoptosis, autophagy, ferroptosis, necroptosis, pyroptosis, and necrosis) are currently considered to be important for discovering new therapeutic opportunities in glioblastoma, which harbors heterogeneity, increasing its ability to adapt to the ever-changing adverse tumor microenvironment. Apoptosis and autophagy maintain cell-membrane integrity and cause no inflammatory response, while all other cell death mechanisms in this list are associated with an ascending order of cell-membrane disruption and thus an increasing order of inflammatory response (mostly an undesirable outcome) after therapy. All of these except necrosis are known to be regulated cell death (RCD) mechanisms. All five RCD mechanisms in this list are now being extensively studied with the aim of modulating their signaling mechanisms using specific therapeutic modalities (chemotherapy, immunotherapy, gene therapy, radiotherapy, photodynamic therapy, sonodynamic therapy, combination therapy, etc.) for preventing the growth of glioblastoma cells and glioblastoma stem cells (GSCs) in preclinical (cell culture and animal) models. Apoptosis (type 1 cell death), autophagic cell death (type 2 cell death), and necrosis (type 3 cell death) are conventionally known to be the most desirable, second-most desirable, and least desirable cell death mechanisms, respectably, in glioblastoma following treatment with a therapeutic modality.
The induction of apoptosis is conventionally the gold standard and the main intention of using a therapy in glioblastoma or in any other cancer. However, all cancers essentially acquire the mechanism of resistance to avoid the induction of apoptosis via alterations in different actors or factors in its complex signaling mechanism in course of the therapy. Tumor microenvironment (e.g., hypoxia, nutrient deficiency, low pH) and some conventional therapies (e.g., chemotherapy, radiotherapy, targeted therapy) promote autophagy, which is basically a mechanism for the bulk degradation of the dysfunctional cellular components (e.g., proteins, nucleic acids, carbohydrates) and the damaged mitochondria and other organelles for producing and recycling the building blocks (i.e., amino acids, nucleotides, sugars), for cellular homeostasis and survival of the tumor cells. Autophagy is a double-edge sword in glioblastoma because it can act as a cell-survival mechanism or a cell-death mechanism depending on the therapeutic strategy used. Studies show that the induction of autophagy inhibits senescence (cessation of cell division) in GSCs, maintaining stemness and promoting senescence in other glioblastoma cells, causing inhibition of their apoptosis, ultimately relapsing the growth of glioblastoma. Therefore, most of the recent therapeutic strategies aim at the induction of apoptosis and inhibition of autophagy, while some of them aim at the induction of autophagic cell death, and a few finding no other viable alternatives aim at the promotion of inflammatory RCD mechanisms in different glioblastoma cells and GSCs.
The non-inflammatory or inflammatory RCD mechanisms encourage the exploration of the efficacy of multiple therapeutic opportunities that need a strong prospect of success not only in preclinical models of glioblastoma but also in clinical trials in glioblastoma patients.
Original research articles of preclinical models and contemporary review articles that directly or indirectly relate to this exciting topic of “Cell Death Mechanisms and Therapeutic Opportunities in Glioblastoma” are cordially invited for submission.
We look forward to receiving your article contributions and working with you for their publication in this Special Issue of Cells.
Prof. Dr. Swapan K. Ray
Guest Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the special issue website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- apoptosis
- autophagy
- cell death mechanisms
- cell signaling
- glioblastoma
- therapeutic opportunities






