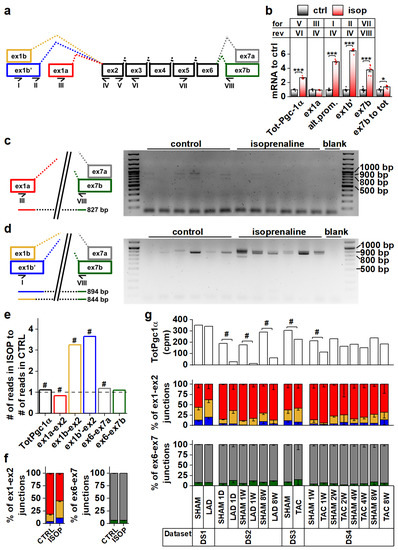
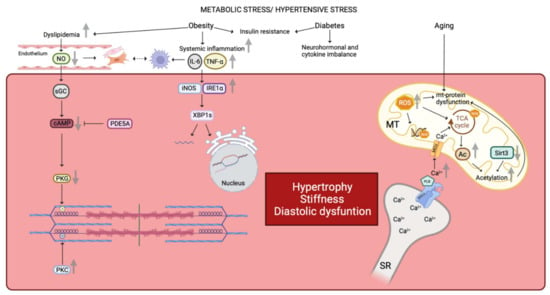
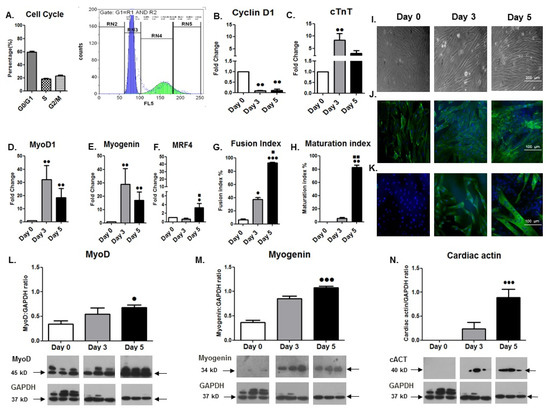
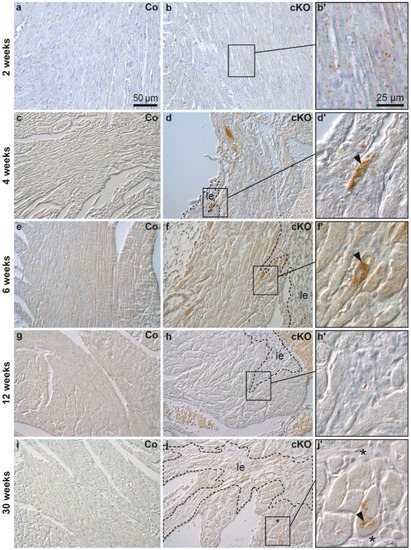
Cardiomyocytes, Myocardial Hypertrophy, and Heart Failure
A topical collection in Cells (ISSN 2073-4409). This collection belongs to the section "Cellular Pathology".
Viewed by 25717Editor
Interests: cardiac hypertrophy; heart failure; cardiomyocytes; metabolism; hypertension
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
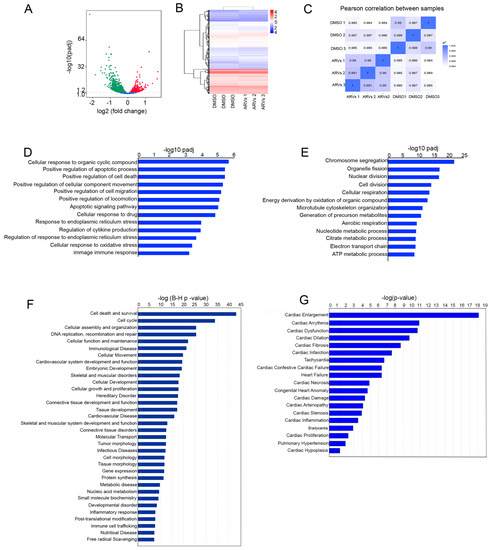
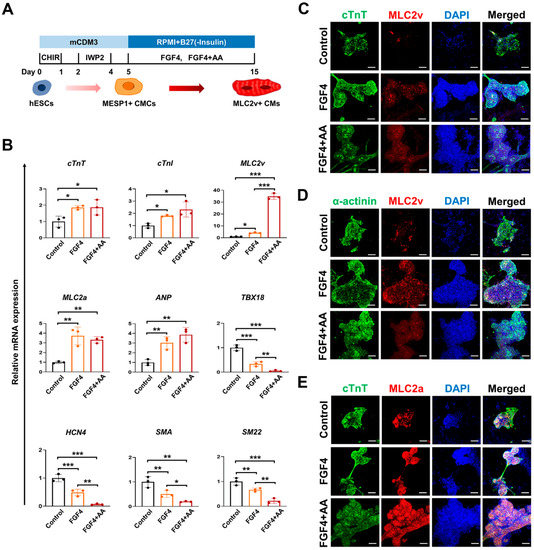
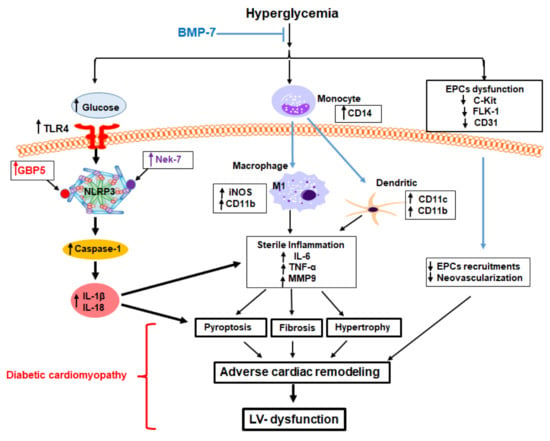
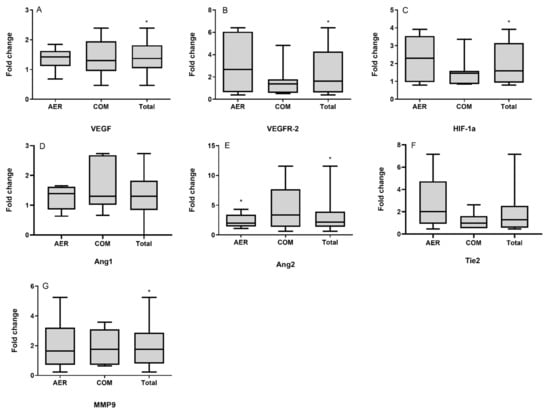
Heart failure is still a leading cause of mortality worldwide. It is caused by cardiomyocytes that lose the ability to generate sufficient force to pump blood into circulation. To improve the current treatment options in cardiology, it is important to have a better understanding of the biological behavior of cardiomyocytes. The function of these cells cannot be completely understood independently of the interaction with surrounding cells, i.e., cardiac fibroblasts and vascular cells. Nevertheless, cardiomyocytes are the cells that have to improve heart work. It is becoming evident that the transition from hypertrophy, an adaptive mechanism to compensate for increased power requirements, to heart failure is the key step to understand how heart function is becoming insufficient. Processes dealing with metabolism, electromechanical coupling, and electrophysiological aspects are among those processes that need to be addressed and understood in their molecular fine regulation in order to improve treatment regimes.
This Topical Collection aims to summarize the current understanding of the process of developing heart failure with a focus on the force-generating cell, the cardiomyocyte.
We look forward to your contributions.
Prof. Dr. Klaus-Dieter SchlüterCollection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- Cardiac metabolism
- Electromechanical coupling
- Regulation of growth and cell death in the heart
- Right heart failure
- Electrophysiological aspects of cardiomyocytes