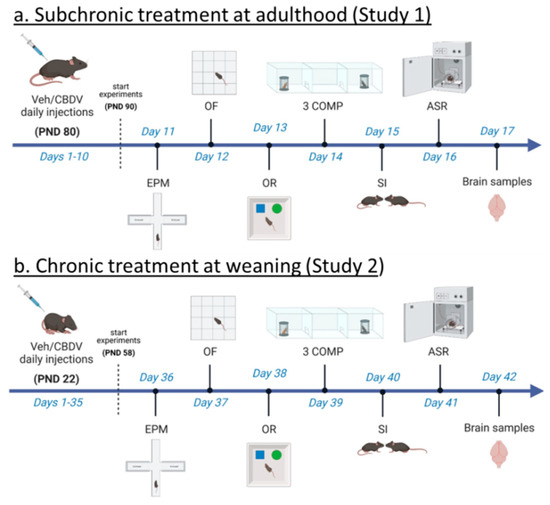
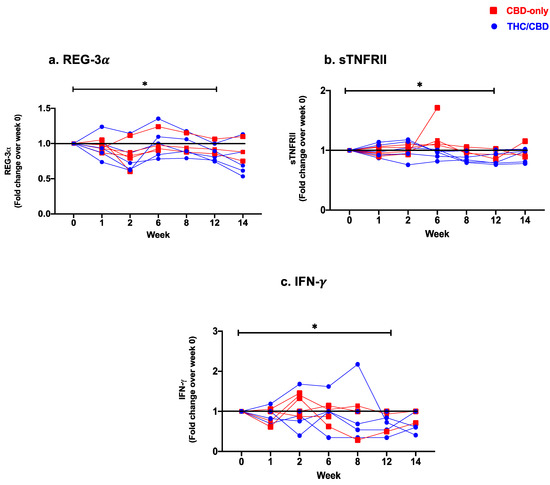
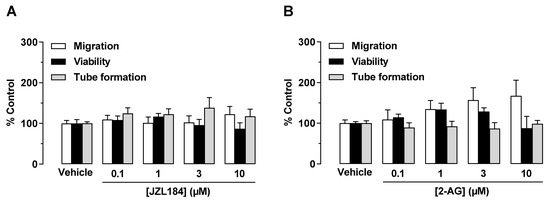
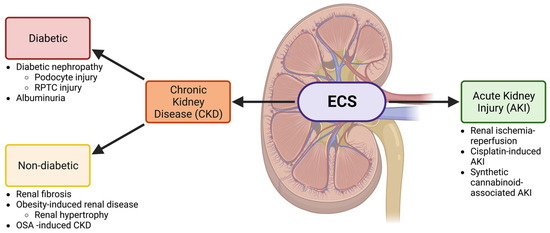
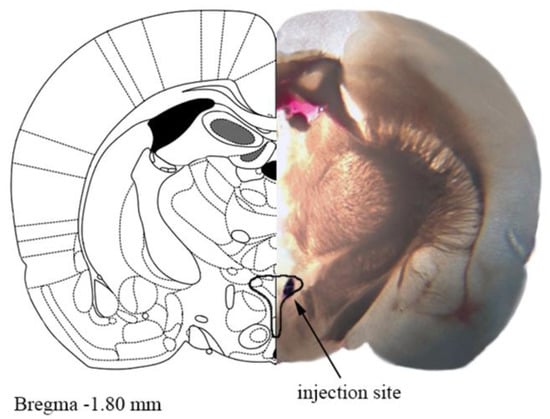
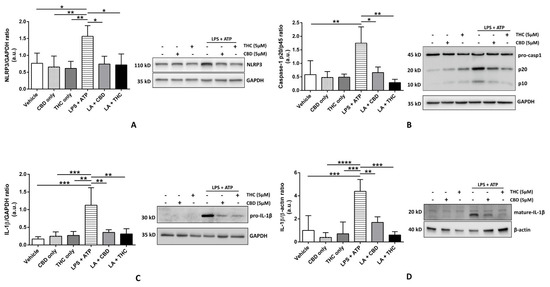
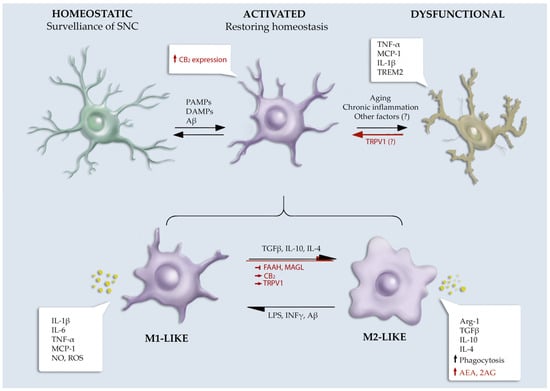
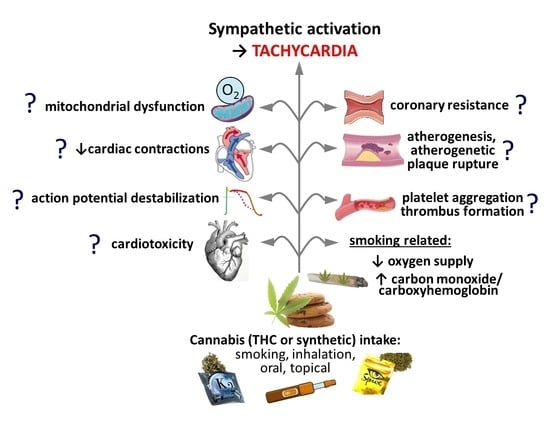
Novel Insights into Cannabinoid Receptors, Molecular Targets, and Therapeutic Potentials
A topical collection in Cells (ISSN 2073-4409). This collection belongs to the section "Cell Signaling".
Viewed by 52981Editor
Topical Collection Information
Dear Colleagues,
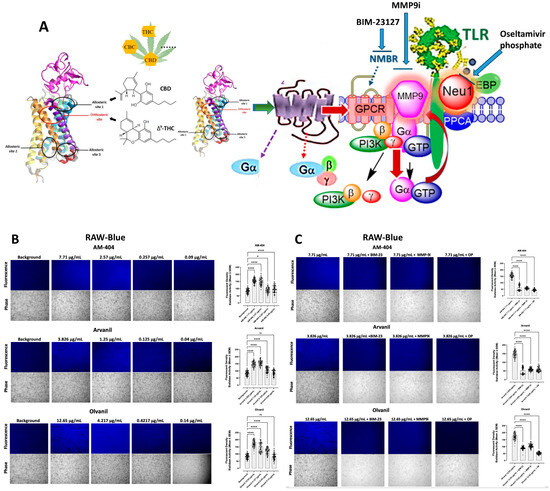
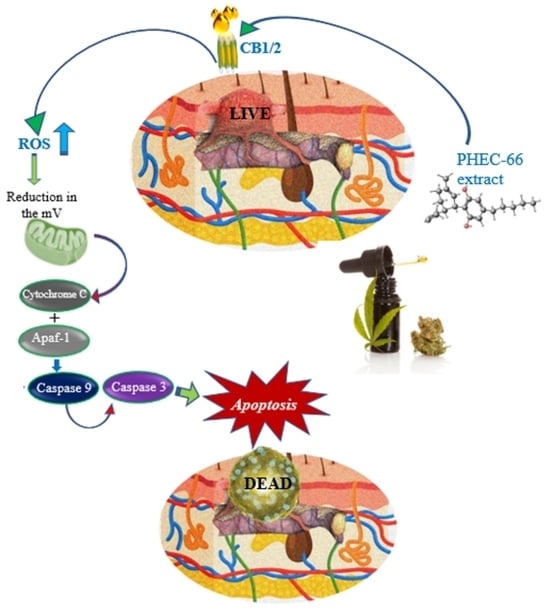
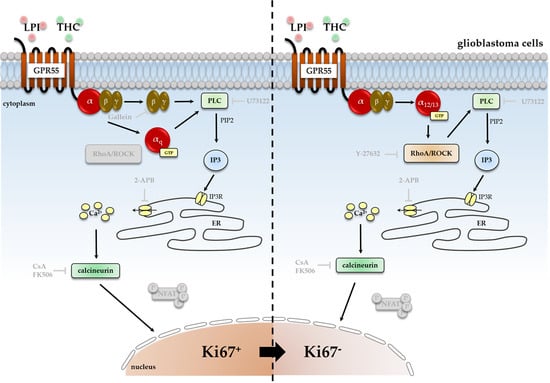
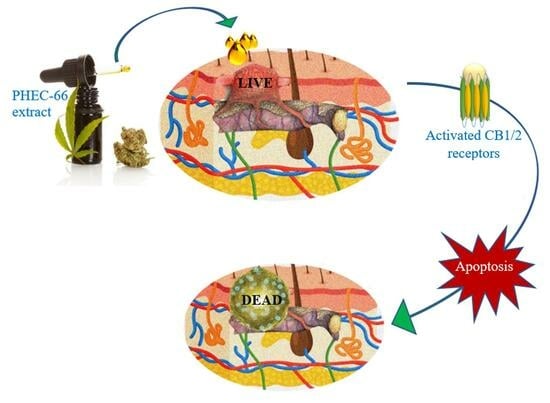
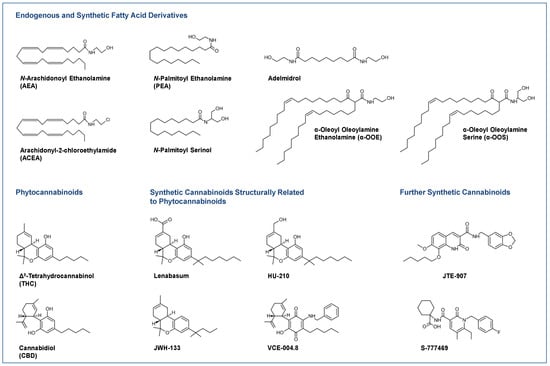
Cannabis has been used as a remedy for illness for centuries in various cultures. Recently, there has been a renewed interest in the uses of cannabis and cannabinoids for medicinal purposes, due to improved legal status in medical cannabis and the advances in cannabinoid research. Cannabinoids are composed of three categories, including phytocannabinoids (the active chemical components of cannabis), endocannabinoids (the cannabinoid-like substances in our body), and synthetic cannabinoids (the cannabinoids prepared in the laboratory). Cannabinoids exert their effects through multiple receptors, targets and signaling pathways. In addition to CB1 and CB2, two well-established cannabinoid receptors, there are numerous molecular targets for cannabinoids, e.g., G-protein-coupled receptors (GPR55, GPR18, GPR3/GPR6/GPR12), transient receptor potential (TRPV) channels, and peroxisome proliferator-activated (PPAR) receptors. These cannabinoid receptors and molecular targets play essential roles for the effects of cannabinoids in health and disease. In addition, they are underscoring the mechanisms of actions for the potential therapeutic effects of a variety of cannabinoids. Recently, there have been tremendous advances in our understanding of these receptors and molecular targets, as well as their implications in the therapeutic potentials of cannabinoids.
The emphasis of this Topical Collection is on the recent advances in our knowledge of cannabinoid receptors, molecular targets, and signaling pathways in the context of physiological/pathological conditions, and cannabinoid therapeutic potentials. Review articles summarizing recent discoveries, and original research articles of both basic and clinical studies are welcome.
We look forward to your important contributions.
Dr. Zhao-Hui Song
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- Cannabinoid receptor
- Molecular target
- Signal transduction
- Therapeutic potentials