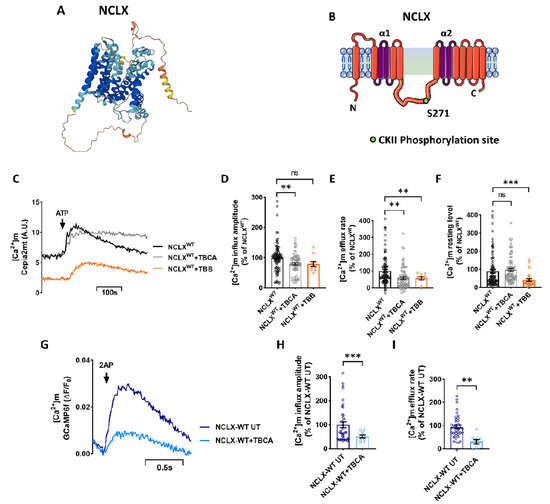
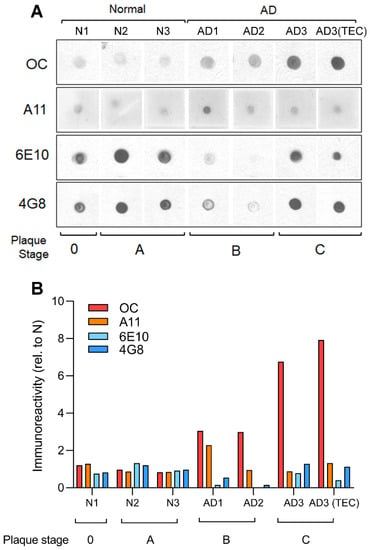
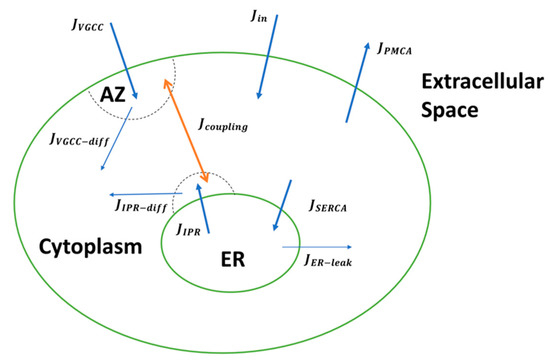
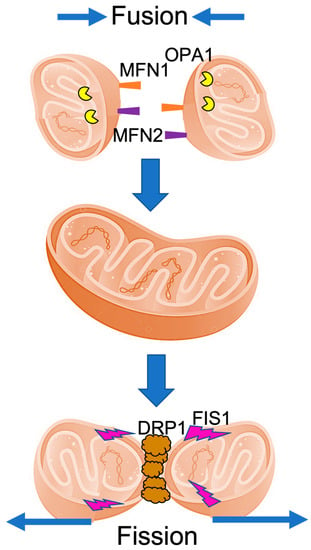
Ca2+ Signaling and Mitochondrial Function in Neurodegenerative Diseases
A topical collection in Cells (ISSN 2073-4409). This collection belongs to the section "Intracellular and Plasma Membranes".
Viewed by 13693Editors
Interests: calcium signaling; mitochondrial function; neurodegenerative diseases; ion channel modeling; ion concentration dynamics; epileptic seizures; spreading depolarization
Interests: calcium signaling; mitochondrial function; neurodegenerative diseases; cytotoxicity; ion channel function; fluorescence microscopy; electrophysiology
Topical Collection Information
Dear Colleagues,
Ca2+ is a ubiquitous second messenger that regulates numerous cell processes, such as synaptic function, memory formation, bioenergetics, and gene transcription. The universality and specificity of Ca2+ signaling stem from its hierarchical spatiotemporal organization, ranging from single channel events to global waves sweeping the entire cell. Substantial evidence accumulated over the last three decades shows that in several neurodegenerative illnesses, including Alzheimer’s, Parkinson’s, and Huntington’s diseases, neuronal and glial Ca2+ signaling is irreversibly disrupted at all these spatiotemporal scales. The uncontrolled rise in cytosolic Ca2+ is believed to cause mitochondrial Ca2+ overload, affecting cell bioenergetics and activating signaling cascade that leads to cell death by apoptosis.
The purpose of this Topic Collection is to overview the current status of the field and highlight the new findings about the role of impaired Ca2+ homeostasis in neurodegenerative diseases and the novel experimental and computational approach now available for their investigation. Special focus will be placed on the role of intracellular oligomeric peptides and intracellular organelles in Ca2+ signaling impairments, Ca2+ mediated changes in mitochondrial function, and the feedback loop between Ca2+ disruptions, intracellular organelles, and amyloidosis.
Dr. Ghanim Ullah
Dr. Angelo Demuro
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- neurodegenerative diseases
- Ca2+ signaling
- mitochondrial function
- intracellular oligomers
- intracellular organelles