The Research of Biomarkers in Colorectal Cancer and Gastric Cancer
A topical collection in Cells (ISSN 2073-4409). This collection belongs to the section "Cellular Pathology".
Viewed by 15985Editors
2. Department of Surgery, Faculty of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan
3. Graduate Institute of Clinical Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan
4. Graduate Institute of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan
5. Center for Cancer Research, Kaohsiung Medical University, Kaohsiung, Taiwan
Interests: precision medicine in colorectal cancer; precision medicine in gastric cancer; cancer biomarkers; molecular oncology
Special Issues, Collections and Topics in MDPI journals
Interests: Colorectal cancer; Oncological nutrition; mini-invasive surgery; surgical oncology; tailored therapy
Interests: adaptive immune system; the major immunological protection mechanism; antigen-specific immune response
Interests: tumor metabolism and epigenetic interplay in gastrointestinal (GI) cancers; KRAS-mutant colorectal cancer; therapeutic targets in GI cancers
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
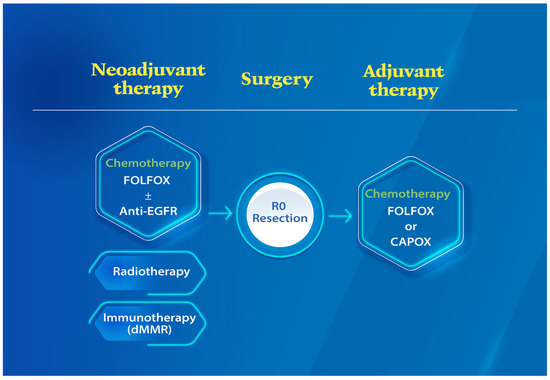
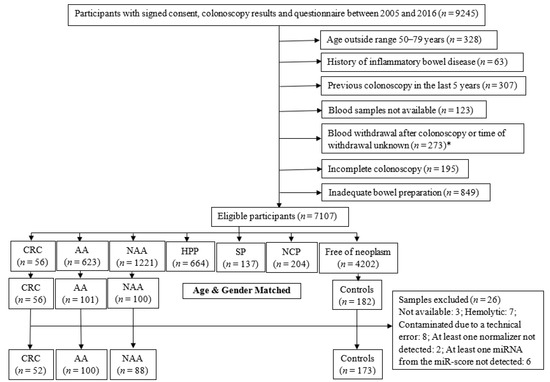
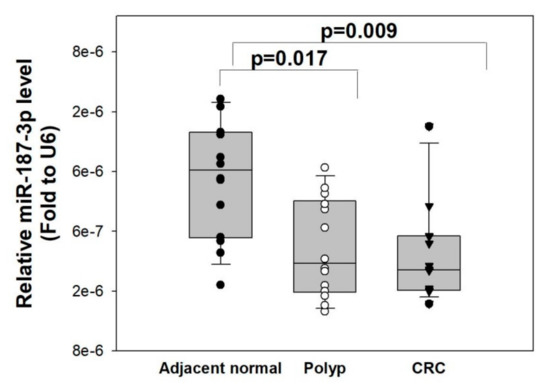
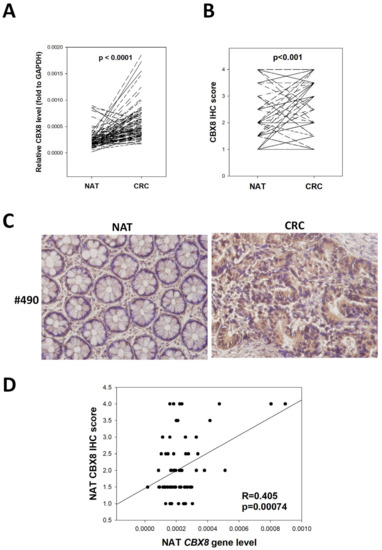
Worldwide, colorectal cancer (CRC) is the second most commonly diagnosed cancer in men and the third most commonly diagnosed in women. In 2018, 1.8 million new CRC cases, with more than 860,000 deaths, were estimated. Based on GLOBOCAN 2018 data, gastric cancer (GC) is the fifth most common neoplasm and the third most deadly cancer, with an estimated 783,000 deaths in 2018. CRC and GC are the two major public health problems worldwide. Early cancer detection, pretherapeutic responsiveness prediction, and an optimal postoperative surveillance strategy are the hallmarks for successful GI cancer treatment. The approval of novel therapies for metastatic GI cancer (mGIC) has led to important improvements in patient outcomes. Despite the multitude of treatments available, outcomes and toxicity with each regimen can vary markedly from patient to patient. Therefore, it is still necessary to increase the individualization of treatments based on tumor genetic profiles to optimize efficacy, while minimizing toxicity. As such, there is currently great focus on the discovery and validation of novel biomarkers in mGIC, with many new potential prognostic and predictive markers being identified alongside developments in molecular profiling technologies. Newer technologies such as ctDNA, miRNA, next-generation sequencing (NGS) and customized genetic panels have highlighted their potential predictive and prognostic roles. There is a clear need for evidence-based recommendations to guide the use of validated and emerging biomarkers in clinical practice. Furthermore, the future focus on the development of emerging biomarkers for the medical unmet needs of GI cancer patients is mandatory.
Prof. Dr. Jaw-Yuan Wang
Guest Editor
Dr. Hsiang-Lin Tsai
Prof. Dr. Hideki Ueno
Prof. Dr. CC. Dennis Wong
Co-Guest Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- biomarkers landscape
- colorectal cancer
- gastric cancer
- personalized medicine
- early detection
- postoperative surveillance