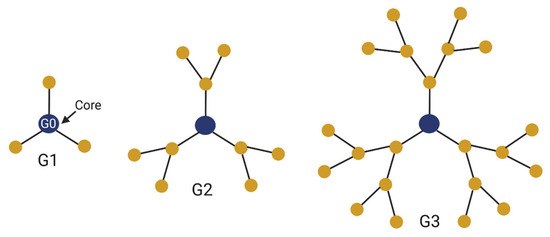
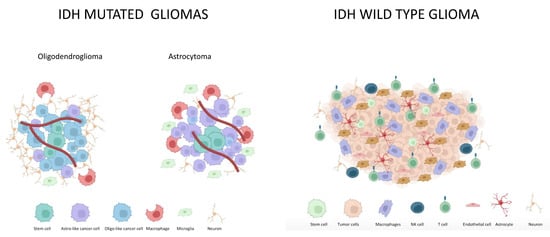
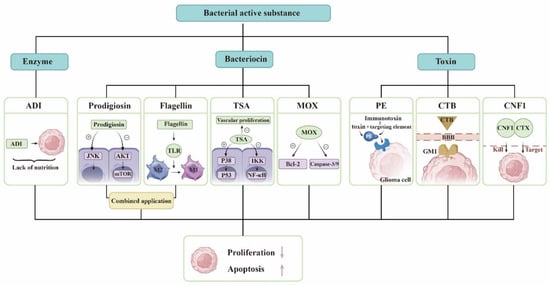
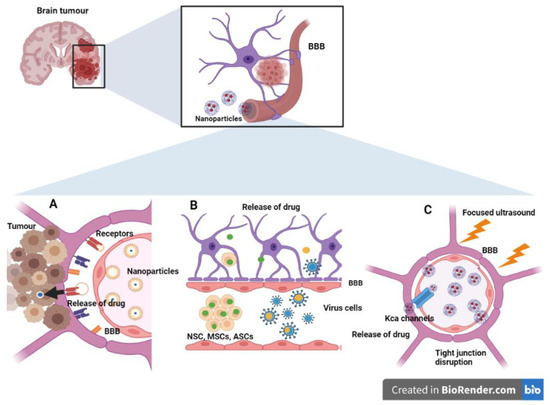
Treatment of Glioma
A topical collection in Cancers (ISSN 2072-6694). This collection belongs to the section "Cancer Therapy".
Viewed by 38973Editors
Interests: glioma
Interests: neurosurgery; glioma; spinal cord injury; neurooncology
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
The management and treatment of adult patients with gliomas present some of the most significant challenges in oncology today. The classification of these tumors has recently undergone major changes, which will bear an impact on treatment and patient monitoring. As a result, new guidelines have recently been developed for the diagnosis and treatment of gliomas and several potential practice-changing clinical trials have recently been completed. Entering the era of molecular diagnostics, personalized chemotherapy and immunotherapy, this Special Issue seeks to give an overview of the field.
This Special Issue aims to give an overview of the latest consensus-recommended conventional treatment paradigms as well as to present new investigational clinical treatment modalities for glioma patients. The intention is also to give an overview of brain tumor rehabilitation programs and the patients' own experiences of treatment as reflected in quality of life assessments.
In this Special Issue, original research articles and reviews are welcome. Research areas may include (but are not limited to) the following: radiological and molecular diagnosis and classification, surgical treatment including laser interstitial therapy, chemotherapy, radiotherapy, tumor-treating fields, immunotherapy, novel drug development, drug repurposing, clinical trials, rehabilitation, neuropsychological aspects, cognition and quality of life.
We look forward to receiving your contributions.
Dr. Johan Bengzon
Dr. Henrietta Nittby
Dr. Safwan Alomari
Guest Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cancers is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2900 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- glioma
- chemotherapy
- radiotherapy
- surgery
- immunotherapy
- clinical trials
- drug development
- rehabilitation
- quality of life