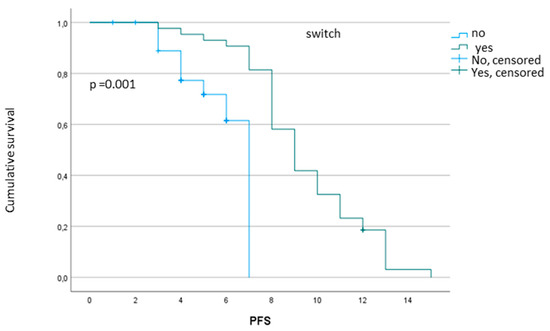
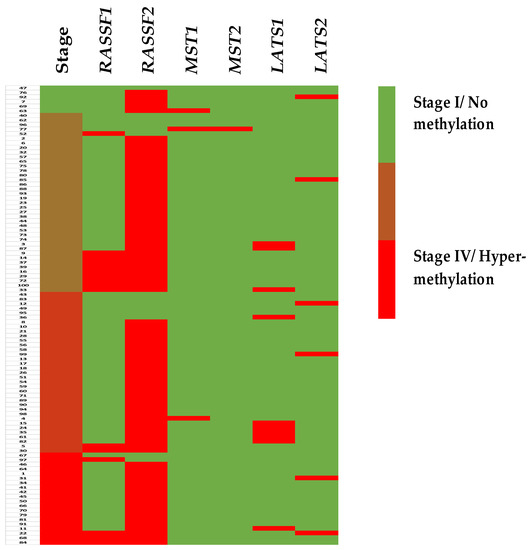
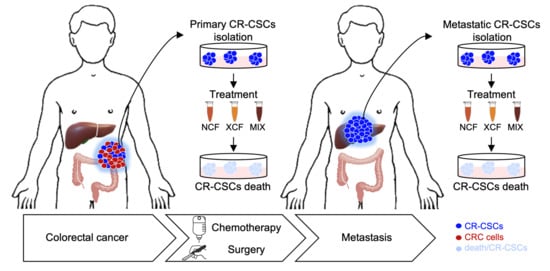
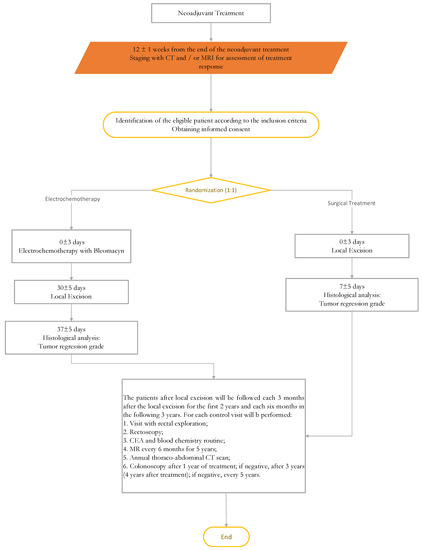
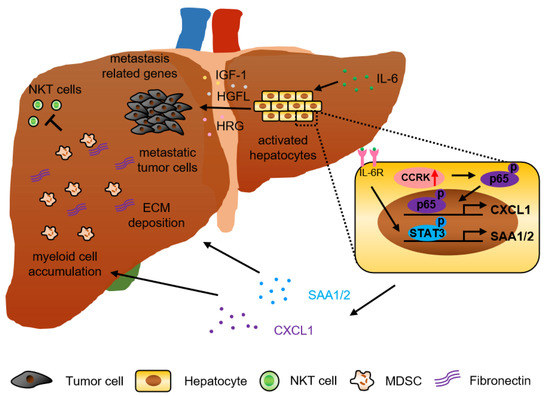
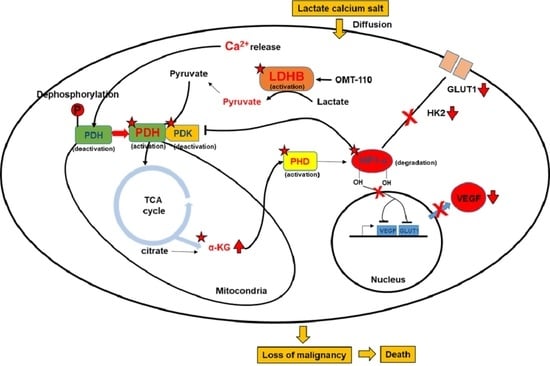
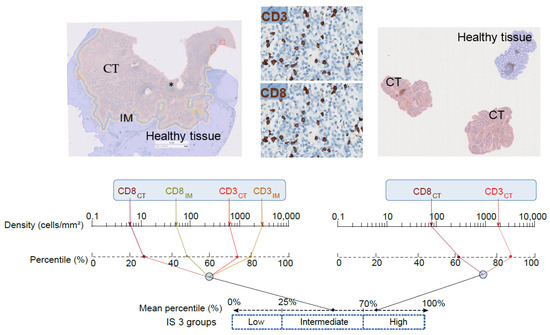
New Treatment for Colorectal Cancer
A topical collection in Cancers (ISSN 2072-6694). This collection belongs to the section "Cancer Therapy".
Viewed by 94940Editor
Interests: colorectal cancer; therapy; inflammation; aging
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
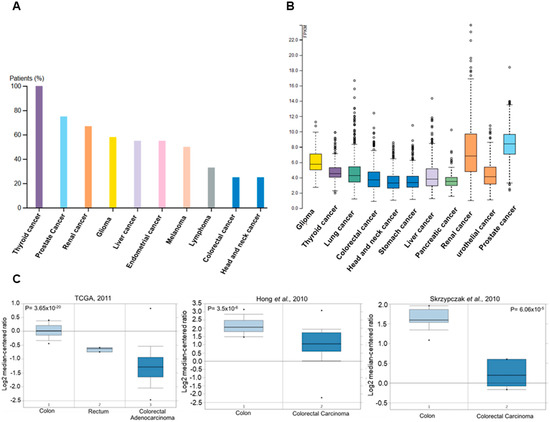
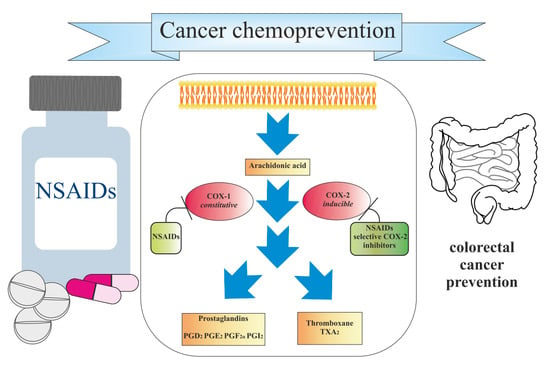
Colorectal cancer incidence rates vary greatly by world region. The disease is considered to be a marker of socioeconomic development and, in countries undergoing a major development transition, incidence rates tend to rise. These increases in incidence have been ascribed to the new western lifestyle, which is correlated with economic improvements, prolonged life expectancy, and aging. There is evidence that processed meat, alcohol, and obesity increase the risk for sporadic colon cancer, whereas physical activity is protective. Epidemiologic studies in the last few decades have noted, in some countries, such as Russia and China, increases in both incidence and mortality. In other countries, such as Canada and the United Kingdom, there has been an increase in incidence but a decrease in mortality. In the United States, Japan, and several western European countries, a reduction in incidence and mortality has been reported. A decrease in incidence has been registered for patients older than 60 years, who represent the majority of patients with colon cancer. Moreover, there has been an increase in incidence and mortality for patients younger than 50 years in the United States and western European countries.
New screening modalities and more effective therapeutic approaches, including specific anti-inflammatory drugs, have the potential to reduce the incidence of mortality due to this common disease.
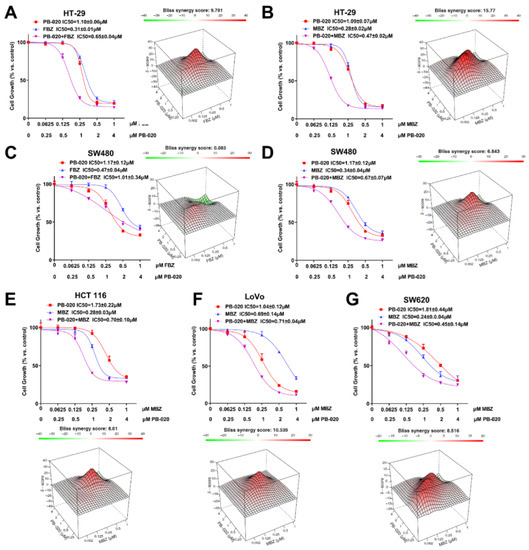
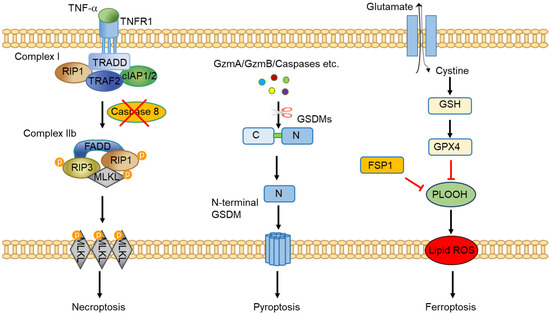
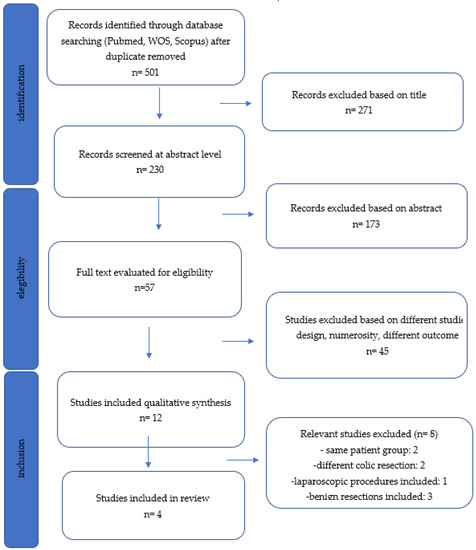
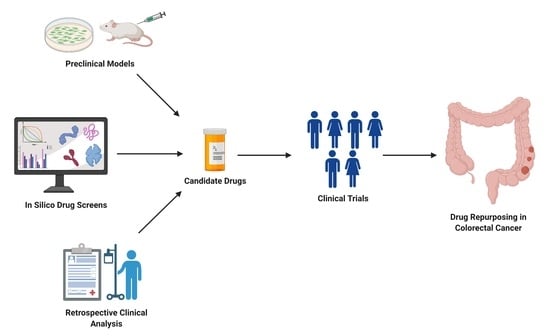
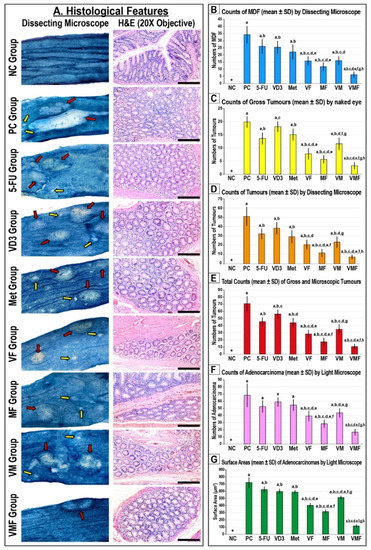
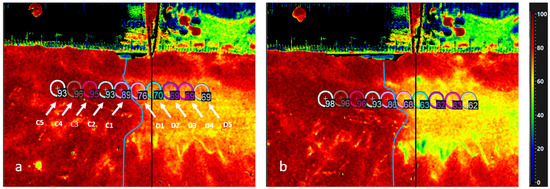
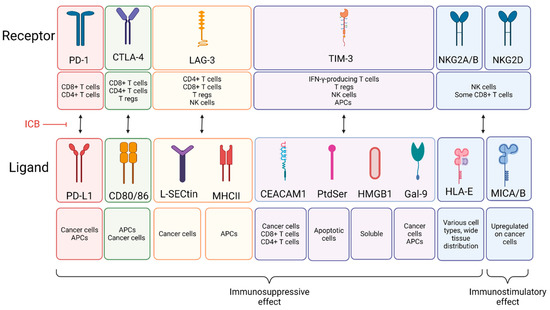
In this Special Issue, we would like to summarize recent advances in colorectal cancer research that may lead to more effective prevention and therapy.
Prof. Antonio V. Sterpetti
Guest Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cancers is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2900 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- colorectal cancer
- therapy
- inflammation
- aging