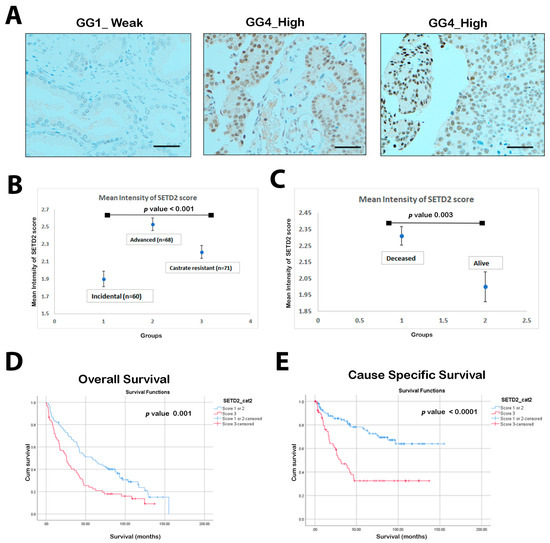
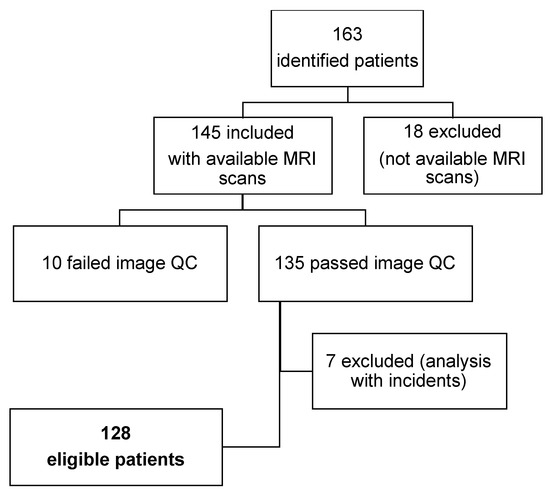
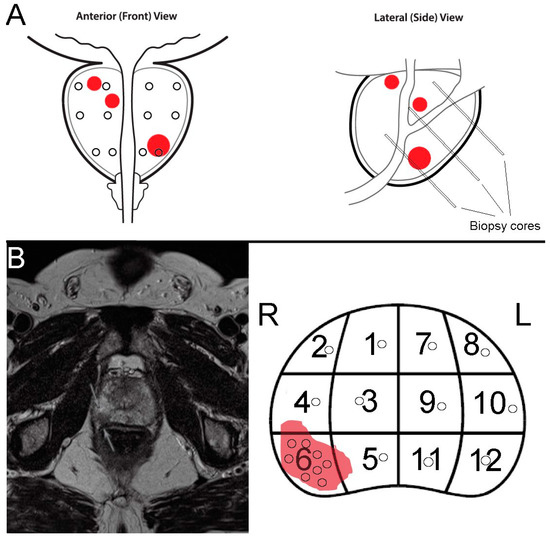
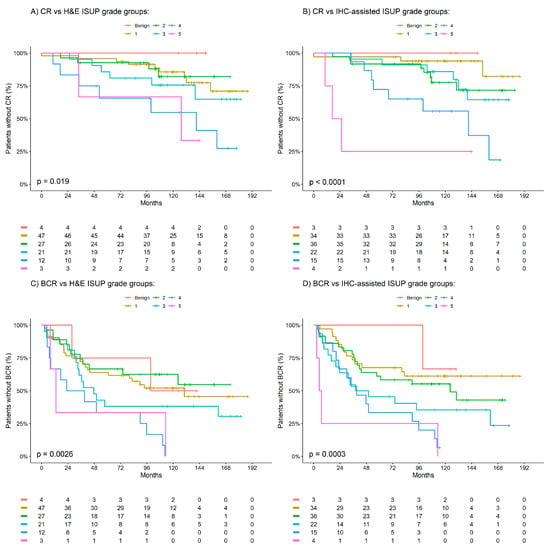
Biomarkers for Detection and Prognosis of Prostate Cancer
A topical collection in Cancers (ISSN 2072-6694). This collection belongs to the section "Cancer Biomarkers".
Viewed by 30426Editor
Interests: biomarkers; prostate cancer; cancer chemoprevention, cancer metabolism; metabolic imaging; drug resistance; castration-resistance; tumor microenvironment; drug repurposing; cancer stem cells
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
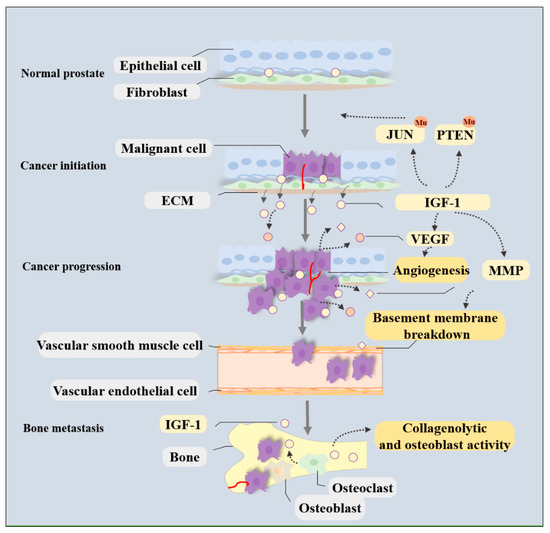
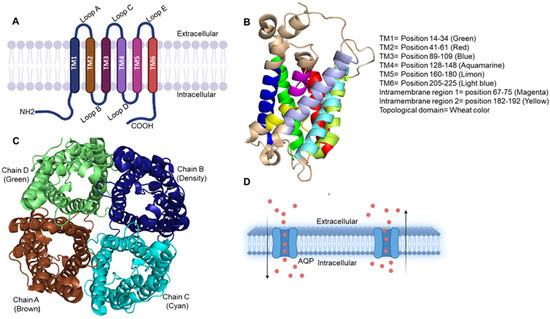
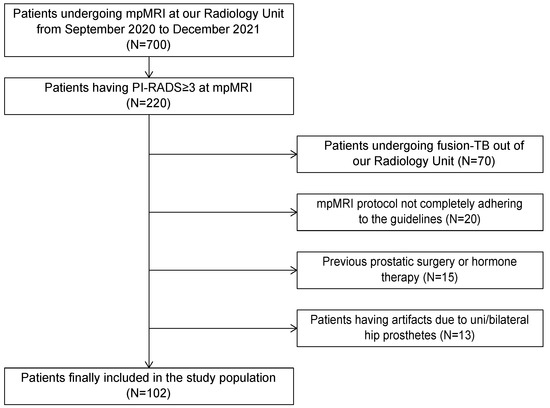
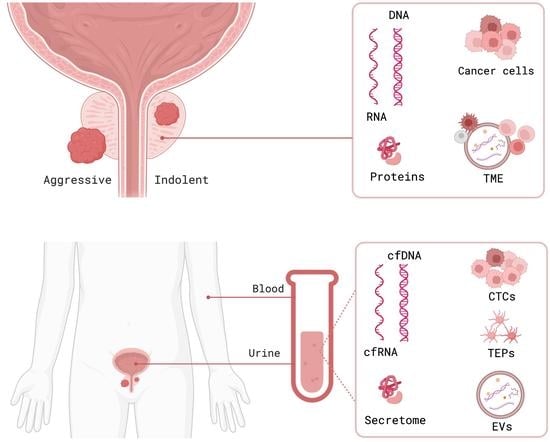
Prostate cancer incidence has been increasing worldwide in recent years. In the United States alone, approximately 250,000 new cases of prostate cancer will be diagnosed and 35,000 men will die from this disease in 2022. Prostate cancer in humans exhibits a unique spectrum of features that include multi-focality, heterogeneity, variable clinical progression, propensity to metastasize to bone, and emergence of androgen-independent forms of the disease. Early detection of prostate cancer rely on serum PSA, which is non-specific biomarker and suffers from high false positive rate. The technical progress in imaging with MRI, and ultrasound scanning, as well as the new urinary and blood-based assays, add a number of components to the process of screening and diagnostic measures.
Approximately 15% of patients with prostate cancer are diagnosed with high-risk disease. To estimate the prognosis of prostate cancer, clinical and pathological scoring systems (TNM classification, grading and staging) are the gold standard and only a few new biomarkers have been useful in the clinical–pathological routine. The recent use of blood and tissue based biomarkers including loss of tumor suppressor genes and rearrangements and fusion detected in prostate cancer provide new prognostic tools as biomarkers of disease progression. Besides invention of new assays and innovative technologies have immensely improved the prediction and prognosis of prostate cancer.
For this Special Issue, I welcome submissions that investigate recent biomarkers in the blood, urine or tumor tissue that may be useful for detection and prognosis of prostate cancer and differentiate between groups of patients at low- and high- risk of biochemical recurrence and disease progression.
Dr. Sanjay Gupta
Guest Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cancers is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2900 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- biomarkers
- prostate cancer
- cancer chemoprevention, cancer metabolism
- metabolic imaging
- drug resistance
- castration-resistance
- tumor microenvironment
- drug repurposing
- cancer stem cells