Advances in the Management of Hepatocellular Carcinoma
A topical collection in Cancers (ISSN 2072-6694). This collection belongs to the section "Cancer Therapy".
Viewed by 58289Editor
Interests: transplantation; surgical oncology; Hepato-Pancreato-Biliary (HPB) surgery
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
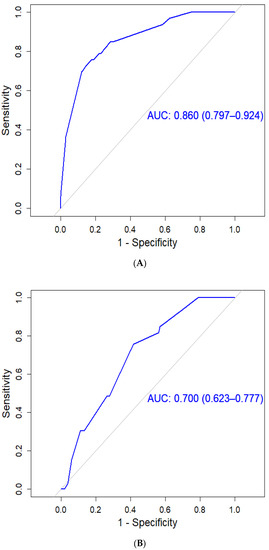
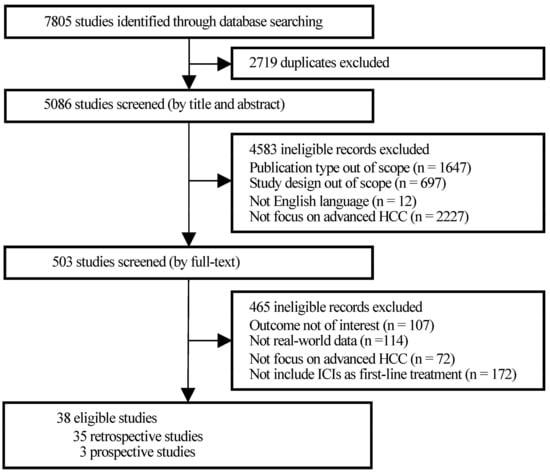
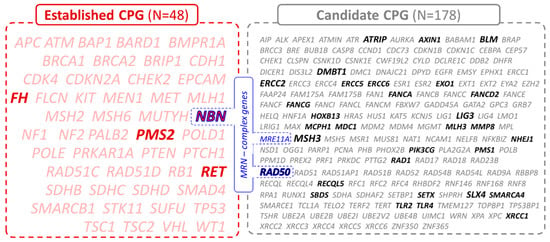
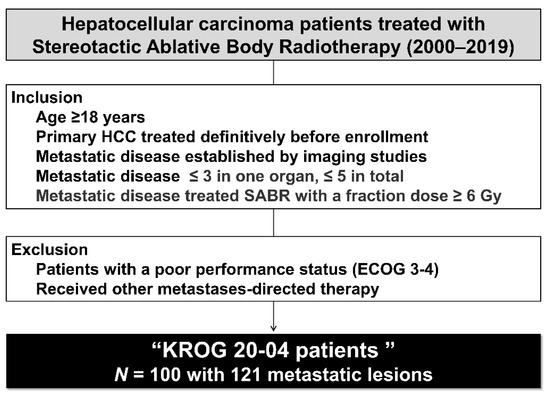
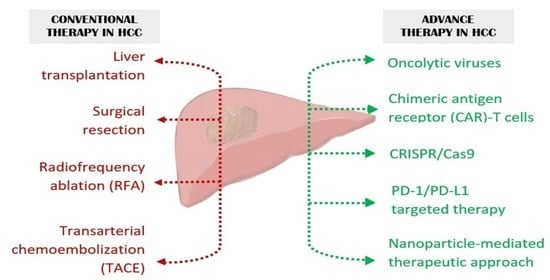
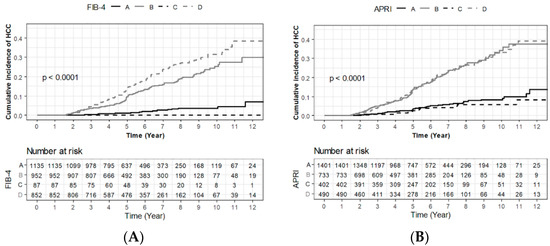
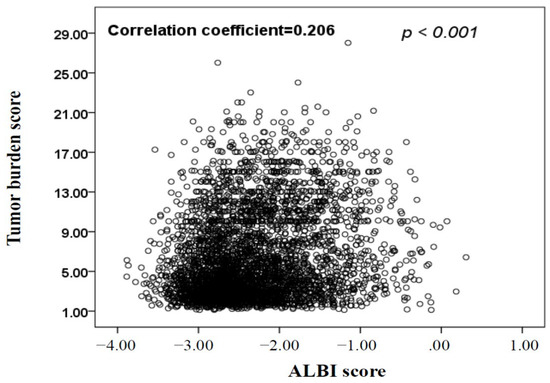
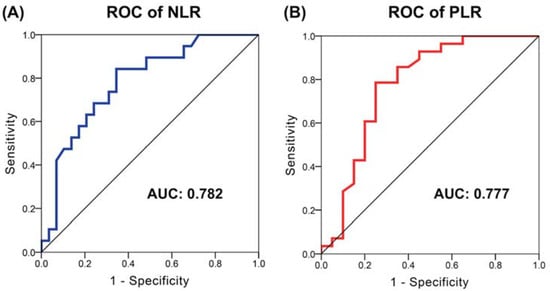
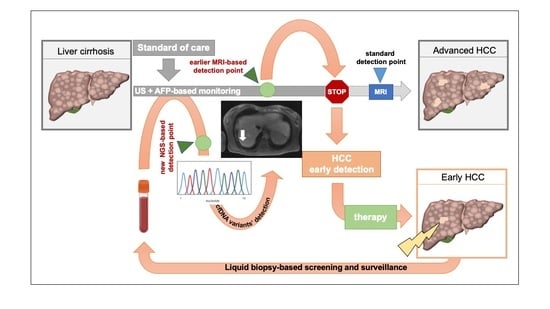
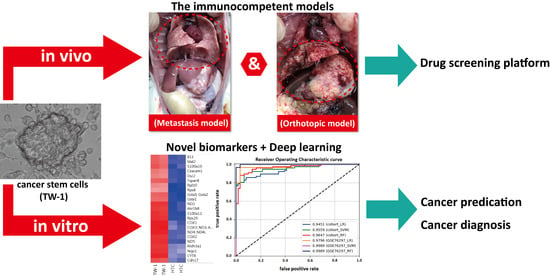
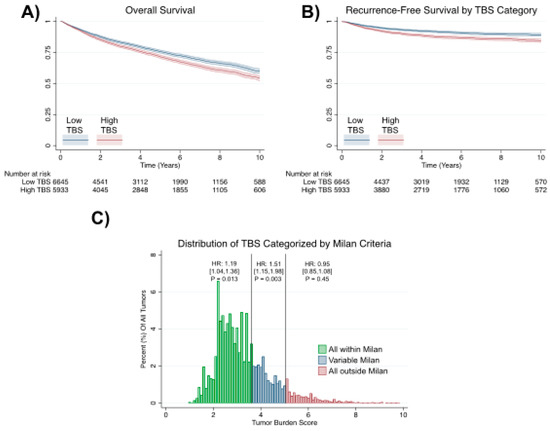
Hepatocellular carcinoma (HCC) is the most common primary liver malignancy, with over 750,000 new cases annually and a concomitant increase in the mortality rate. Despite prevention and screening efforts, as well as the development of new technologies for diagnosis and treatment, the incidence of HCC has doubled, and mortality rates have increased in recent decades. Multiple treatment modalities have been proposed for HCC. Liver transplantation (LT) and surgical resection with curative potential are currently the primary treatments. Selection of treatment modality is based on tumor size, tumor location, extrahepatic spread, and underlying liver function. Patients undergoing LT or surgical resection achieve a long-term survival rate of more than 50% at 5 years; however, only a small group of patients with early stage HCC are eligible for these therapies.
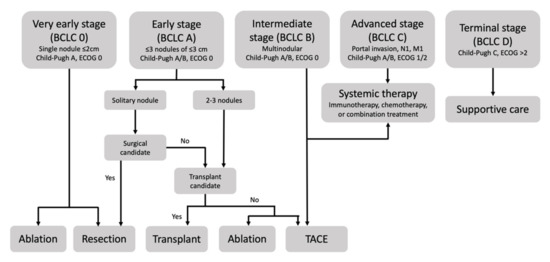
Among the staging systems available, the Barcelona Clinic Liver Cancer (BCLC) system has been used widely in the West. According to the BCLC system, resection should be offered only to patients with BCLC-0 and BCLC-A HCC. Nevertheless, these criteria have been considered to be too vague and restrictive, with studies showing heterogeneous outcomes after resection of HCC within the same stage. In addition, several investigators have recently advocated for extending the criteria for resection, acknowledging that certain patients with BCLC-B HCC may benefit more from surgery than other locoregional therapies. The prognostic discrimination as well as the treatment allocation of the revised BCLC classification have been questioned, emphasizing the need for refinement and further subclassification of this system.
Dr. Dimitrios Moris
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cancers is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2900 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- hepatocellular carcinoma
- BCLC
- resection
- transplantation
- immunotherapy
- chemotherapy