Feature Papers in Molecular Reproduction
Share This Topical Collection
Editor
Topical Collection Information
Dear Colleagues,
This Topical Collection, “Feature Papers in Molecular Reproduction”, will bring together high-quality research articles, review articles, and communications on the subject of molecular reproductive biology and medicine, reproductive and developmental biology (including reproductive immunology), and the molecular diagnosis of human reproductive diseases.
The Topical Collection is dedicated to diverse recent advances in reproduction research, as highlighted in the topics below, and comprises a selection of exclusive papers from the Editorial Board Members of the Molecular Reproduction Section as well as invited papers from relevant experts. We also welcome established experts in the field to make contributions to this Topical Collection. Please note that all invited papers will be published online once accepted. We aim to represent our Section as an attractive open-access publishing platform for molecular reproduction research.
Topics include, without being limited to:
- Reproductive biology;
- Reproductive immunology;
- Reproductive medicine;
- Reproductive endocrinology;
- Molecular regulation of reproductive processes;
- Embryo development;
- New techniques and methods in reproductive biology;
- Gametogenesis and fertilization
Dr. Mingqing Li
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Biomolecules is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs).
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Published Papers (9 papers)
Open AccessArticle
Dysbiosis of Gut Microbiome Aggravated Male Infertility in Captivity of Plateau Pika
by
Liangzhi Zhang, Xianjiang Tang, Chao Fan, Shi’en Ren, Qi Cheng, Huakun Zhou, Kai Liu, Shangang Jia and Yanming Zhang
Viewed by 477
Abstract
Captivity is an important and efficient technique for rescuing endangered species. However, it induces infertility, and the underlying mechanism remains obscure. This study used the plateau pika (
Ochotona curzoniae) as a model to integrate physiological, metagenomic, metabolomic, and transcriptome analyses and
[...] Read more.
Captivity is an important and efficient technique for rescuing endangered species. However, it induces infertility, and the underlying mechanism remains obscure. This study used the plateau pika (
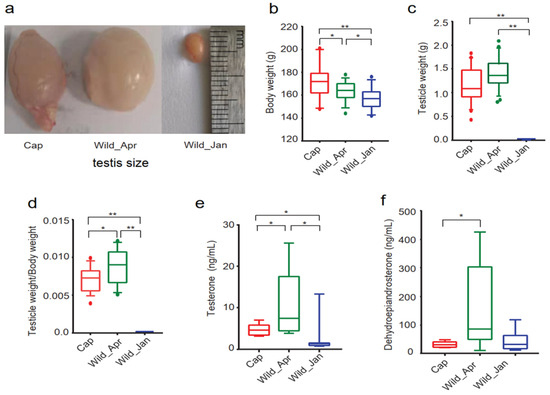
Ochotona curzoniae) as a model to integrate physiological, metagenomic, metabolomic, and transcriptome analyses and explore whether dysbiosis of the gut microbiota induced by artificial food exacerbates infertility in captive wild animals. Results revealed that captivity significantly decreased testosterone levels and the testicle weight/body weight ratio. RNA sequencing revealed abnormal gene expression profiles in the testicles of captive animals. The microbial α-diversity and Firmicutes/Bacteroidetes ratio were drastically decreased in the captivity group.
Bacteroidetes and
Muribaculaceae abundance notably increased in captive pikas. Metagenomic analysis revealed that the alteration of flora increased the capacity for carbohydrate degradation in captivity. The levels of microbe metabolites’ short-chain fatty acids (SCFAs) were significantly high in the captive group. Increasing SCFAs influenced the immune response of captivity plateau pikas; pro-inflammatory cytokines were upregulated in captivity. The inflammation ultimately contributed to male infertility. In addition, a positive correlation was observed between
Gastranaerophilales family abundance and testosterone concentration. Our results provide evidence for the interactions between artificial food, the gut microbiota, and male infertility in pikas and benefit the application of gut microbiota interference in threatened and endangered species.
Full article
►▼
Show Figures
Open AccessReview
Current Status and Future Prospects of Stem Cell Therapy for Infertile Patients with Premature Ovarian Insufficiency
by
Hye Kyeong Kim and Tae Jin Kim
Viewed by 1529
Abstract
Premature ovarian insufficiency (POI), also known as premature menopause or premature ovarian failure, signifies the partial or complete loss of ovarian endocrine function and fertility before 40 years of age. This condition affects approximately 1% of women of childbearing age. Although 5–10% of
[...] Read more.
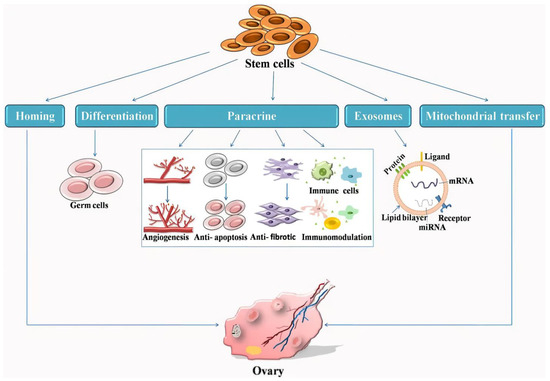
Premature ovarian insufficiency (POI), also known as premature menopause or premature ovarian failure, signifies the partial or complete loss of ovarian endocrine function and fertility before 40 years of age. This condition affects approximately 1% of women of childbearing age. Although 5–10% of patients may conceive naturally, conventional infertility treatments, including assisted reproductive technology, often prove ineffective for the majority. For infertile patients with POI, oocyte donation or adoption exist, although a prevalent desire persists among them to have biological children. Stem cells, which are characterized by their undifferentiated nature, self-renewal capability, and potential to differentiate into various cell types, have emerged as promising avenues for treating POI. Stem cell therapy can potentially reverse the diminished ovarian endocrine function and restore fertility. Beyond direct POI therapy, stem cells show promise in supplementary applications such as ovarian tissue cryopreservation and tissue engineering. However, technological and ethical challenges hinder the widespread clinical application of stem cells. This review examines the current landscape of stem cell therapy for POI, underscoring the importance of comprehensive assessments that acknowledge the diversity of cell types and functions. Additionally, this review scrutinizes the limitations and prospects associated with the clinical implementation of stem cell treatments for POI.
Full article
►▼
Show Figures
Open AccessArticle
New Insights on Sperm Function in Male Infertility of Unknown Origin: A Multimodal Approach
by
Rita I. Pacheco, Maria I. Cristo, Sandra I. Anjo, Andreia F. Silva, Maria Inês Sousa, Renata S. Tavares, Ana Paula Sousa, Teresa Almeida Santos, Mariana Moura-Ramos, Francisco Caramelo, Bruno Manadas, João Ramalho-Santos and Sandra Gomes Amaral
Viewed by 1896
Abstract
The global trend of rising (male) infertility is concerning, and the unidentifiable causes in half of the cases, the so-called unknown origin male infertility (UOMI), demands a better understanding and assessment of both external/internal factors and mechanisms potentially involved. In this work, it
[...] Read more.
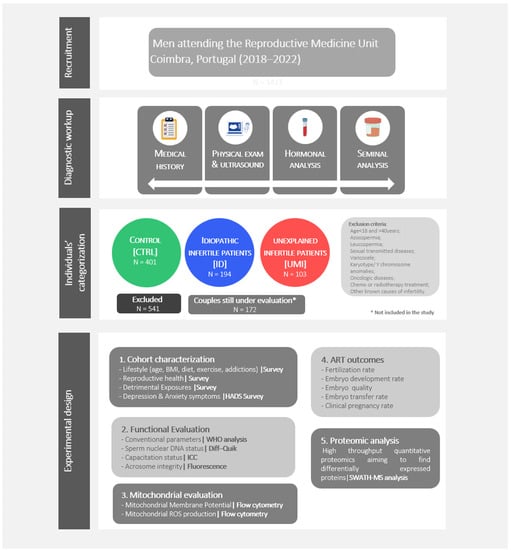
The global trend of rising (male) infertility is concerning, and the unidentifiable causes in half of the cases, the so-called unknown origin male infertility (UOMI), demands a better understanding and assessment of both external/internal factors and mechanisms potentially involved. In this work, it was our aim to obtain new insight on UOMI, specifically on idiopathic (ID) and Unexplained male infertility (UMI), relying on a detailed evaluation of the male gamete, including functional, metabolic and proteomic aspects. For this purpose, 1114 semen samples, from males in couples seeking infertility treatment, were collected at the Reproductive Medicine Unit from the Centro Hospitalar e Universitário de Coimbra (CHUC), from July 2018–July 2022. Based on the couples’ clinical data, seminal/hormonal analysis, and strict eligibility criteria, samples were categorized in 3 groups, control (CTRL), ID and UMI. Lifestyle factors and anxiety/depression symptoms were assessed via survey. Sperm samples were evaluated functionally, mitochondrially and using proteomics. The results of Assisted Reproduction Techniques were assessed whenever available. According to our results, ID patients presented the worst sperm functional profile, while UMI patients were similar to controls. The proteomic analysis revealed 145 differentially expressed proteins, 8 of which were specifically altered in ID and UMI samples. Acrosin (ACRO) and sperm acrosome membrane-associated protein 4 (SACA4) were downregulated in ID patients while laminin subunit beta-2 (LAMB2), mannose 6-phosphate isomerase (MPI), ATP-dependent 6-phosphofructokinase liver type (PFKAL), STAR domain-containing protein 10 (STA10), serotransferrin (TRFE) and exportin-2 (XPO2) were downregulated in UMI patients. Using random forest analysis, SACA4 and LAMB2 were identified as the sperm proteins with a higher chance of distinguishing ID and UMI patients, and their function and expression variation were in accordance with the functional results. No alterations were observed in terms of lifestyle and psychological factors among the 3 groups. These findings obtained in an experimental setting based on 3 well-defined groups of subjects, might help to validate new biomarkers for unknown origin male infertility (ID and UMI) that, in the future, can be used to improve diagnostics and treatments.
Full article
►▼
Show Figures
Open AccessBrief Report
SARS-CoV-2 Infection in Unvaccinated High-Risk Pregnant Women in the Bronx, NY, USA Is Associated with Decreased Apgar Scores and Placental Villous Infarcts
by
Sandra E. Reznik, Patricia M. Vuguin, Alexa Cohen, Rasha Khoury, Olivier Loudig, Ridin Balakrishnan, Susan A. Fineberg, Francine Hughes, Malini Harigopal and Maureen J. Charron
Viewed by 1505
Abstract
Babies born to severe acute respiratory syndrome corona virus-2 (SARS-CoV-2)-infected mothers are at greater risk for perinatal morbidity and more likely to receive a neurodevelopmental diagnosis in the first year of life. However, the effect of maternal infection on placental function and neonatal
[...] Read more.
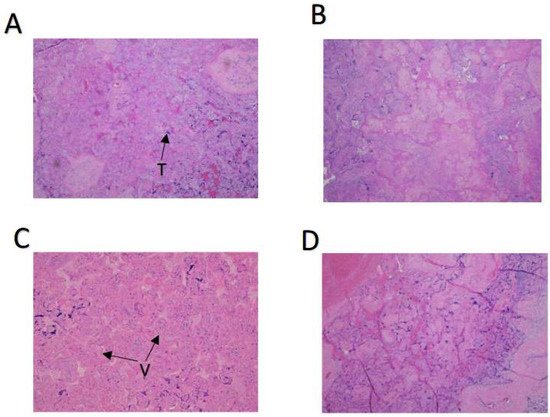
Babies born to severe acute respiratory syndrome corona virus-2 (SARS-CoV-2)-infected mothers are at greater risk for perinatal morbidity and more likely to receive a neurodevelopmental diagnosis in the first year of life. However, the effect of maternal infection on placental function and neonatal outcomes varies depending upon the patient population. We set out to test our hypothesis that maternal SARS-CoV-2 infection in our underserved, socioeconomically disadvantaged, mostly unvaccinated, predominantly African American and Latina population in the Bronx, NY would have effects evident at birth. Under IRB approval, 56 SARS-CoV-2-positive patients infected during the “first wave” of the pandemic with alpha and beta strains of the virus, 48 patients infected during the “second wave” of the pandemic with delta and omicron strains and 61 negative third-trimester high-risk patients were randomly selected from Montefiore Medical Center (MMC), Bronx, NY. In addition, two positive cases from Yale New Haven Hospital, CT were included as controls. All 104 placentas delivered by SARS-CoV-2-positive mothers were uninfected by the virus, based on immunohistochemistry, in situ hybridization, and qPCR analysis. However, placental villous infarcts were significantly increased in first-wave cases compared to second-wave cases or negative controls. Significantly lower Apgar scores at 1 min and 5 min were observed in neonates born to infected mothers with severe symptoms. These findings suggest that even without entering the placenta, SARS-CoV-2 can affect various systemic pathways, culminating in altered placental development and function, which may adversely affect the fetus, especially in a high-risk patient population such as ours. These results underline the importance of vaccination among pregnant women, particularly in low-resource areas.
Full article
►▼
Show Figures
Open AccessArticle
RETRACTED: Palmitic Acid Impedes Extravillous Trophoblast Activity by Increasing MRP1 Expression and Function
by
Yunali Ashar, Qiuxu Teng, John N. D. Wurpel, Zhe-Sheng Chen and Sandra E. Reznik
Cited by 1 | Viewed by 2237
|
Retraction
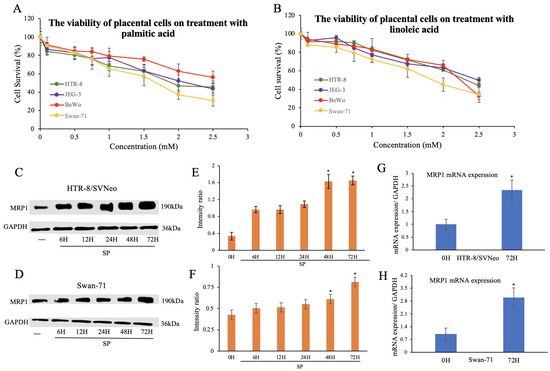
Abstract
Normal function of placental extravillous trophoblasts (EVTs), which are responsible for uteroplacental vascular remodeling, is critical for adequate delivery of oxygen and nutrients to the developing fetus and normal fetal programming. Proliferation and invasion of spiral arteries by EVTs depends upon adequate levels
[...] Read more.
Normal function of placental extravillous trophoblasts (EVTs), which are responsible for uteroplacental vascular remodeling, is critical for adequate delivery of oxygen and nutrients to the developing fetus and normal fetal programming. Proliferation and invasion of spiral arteries by EVTs depends upon adequate levels of folate. Multidrug resistance-associated protein 1 (MRP1), which is an efflux transporter, is known to remove folate from these cells. We hypothesized that palmitic acid increases MRP1-mediated folate removal from EVTs, thereby interfering with EVTs’ role in early placental vascular remodeling. HTR-8/SVneo and Swan-71 cells, first trimester human EVTs, were grown in the absence or presence of 0.5 mM and 0.7 mM palmitic acid, respectively, for 72 h. Palmitic acid increased
ABCC1 gene expression and MRP1 protein expression in both cell lines. The rate of folate efflux from the cells into the media increased with a decrease in migration and invasion functions in the cultured cells. Treatment with N-acetylcysteine (NAC) prevented the palmitic acid-mediated upregulation of MRP1 and restored invasion and migration in the EVTs. Finally, in an
ABCC1 knockout subline of Swan-71 cells, there was a significant increase in invasion and migration functions. The novel finding in this study that palmitic acid increases MRP1-mediated folate efflux provides a missing link that helps to explain how maternal consumption of saturated fatty acids compromises the in utero environment.
Full article
►▼
Show Figures
Open AccessEditor’s ChoiceArticle
Gal-2 Increases H3K4me3 and H3K9ac in Trophoblasts and Preeclampsia
by
Laura Hahn, Sarah Meister, Mareike Mannewitz, Susanne Beyer, Stefanie Corradini, Uwe Hasbargen, Sven Mahner, Udo Jeschke, Thomas Kolben and Alexander Burges
Cited by 2 | Viewed by 2004
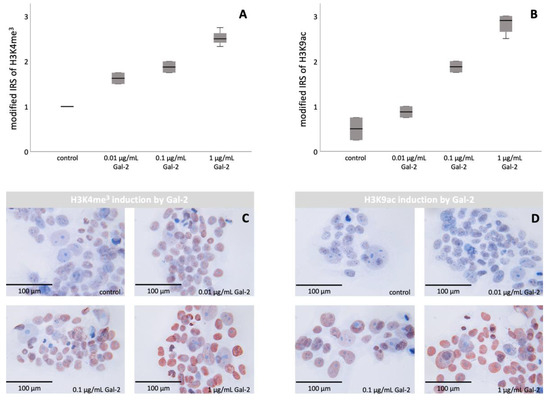
Abstract
Preeclampsia (PE) is a severe pregnancy disorder with a pathophysiology not yet completely understood and without curative therapy. The histone modifications H3K4me
3 and H3K9ac, as well as galectin-2 (Gal-2), are known to be decreased in PE. To gain a better understanding of
[...] Read more.
Preeclampsia (PE) is a severe pregnancy disorder with a pathophysiology not yet completely understood and without curative therapy. The histone modifications H3K4me
3 and H3K9ac, as well as galectin-2 (Gal-2), are known to be decreased in PE. To gain a better understanding of the development of PE, the influence of Gal-2 on histone modification in trophoblasts and in syncytialisation was investigated. Immunohistochemical stains of 13 PE and 13 control placentas were correlated, followed by cell culture experiments. An analysis of H3K4me
3 and H3K9ac was conducted, as well as cell fusion staining with E-cadherin and β-catenin—both after incubation with Gal-2. The expression of H3K4me
3 and H3K9ac correlated significantly with the expression of Gal-2. Furthermore, we detected an increase in H3K4me
3 and H3K9ac after the addition of Gal-2 to BeWo/HVT cells. Moreover, there was increased fusion of HVT cells after incubation with Gal-2. Gal-2 is associated with the histone modifications H3K4me
3 and H3K9ac in trophoblasts. Furthermore, syncytialisation increased after incubation with Gal-2. Therefore, we postulate that Gal-2 stimulates syncytialisation, possibly mediated by H3K4me
3 and H3K9ac. Since Gal-2, as well as H3K4me
3 and H3K9ac, are decreased in PE, the induction of Gal-2 might be a promising therapeutic target.
Full article
►▼
Show Figures
Open AccessArticle
Progesterone Attenuates SIRT1-Deficiency-Mediated Pre-Eclampsia
by
Jiangnan Pei, Zhenzhen Liu, Chengjie Wang, Nan Chu, Lei Liu, Yao Tang, Haiyan Liu, Qianqian Xiang, Haidong Cheng, Mingqing Li and Weirong Gu
Cited by 12 | Viewed by 3289
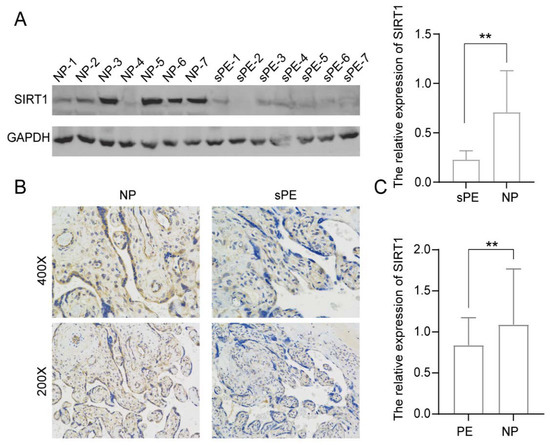
Abstract
Pre-eclampsia is a severe hypertensive disorder of pregnancy (HDP), mainly characterized by new-onset hypertension with proteinuria after 20-week gestation. Sirtuin1 (SIRT1), a class III histone deacetylase, is associated with the regulation of various pathophysiological processes, including inflammation, immune response, metabolism, and autophagy. However,
[...] Read more.
Pre-eclampsia is a severe hypertensive disorder of pregnancy (HDP), mainly characterized by new-onset hypertension with proteinuria after 20-week gestation. Sirtuin1 (SIRT1), a class III histone deacetylase, is associated with the regulation of various pathophysiological processes, including inflammation, immune response, metabolism, and autophagy. However, the effect of SIRT1 in the pathogenesis of pre-eclampsia remains to be elucidated. In this study, we found that the expression of SIRT1 was relatively lower in the placentas and serum samples of pre-eclampsia patients. Typical pre-eclampsia-like symptoms, such as hypertension, proteinuria, fetal growth restriction, kidney injury, and a narrow placental labyrinth layer, were observed in SIRT1 knockdown (SIRT1
+/−) mice. Of note, these performances could be improved after the intraperitoneal injection of SIRT1 agonist SRT2104. More importantly, we found that the efficacy of progesterone on attenuating symptoms of PE was profoundly better than that of metformin in SIRT1
+/− mice. In addition, our results suggested that progesterone can promote the invasion and inhibit the apoptosis of trophoblasts. These data suggest that SIRT1 plays an important role in pre-eclampsia and that progesterone alleviates pre-eclampsia-like symptoms mediated by SIRT1 deficiency.
Full article
►▼
Show Figures
Open AccessArticle
Single-Cell Sequencing Reveals an Intrinsic Heterogeneity of the Preovulatory Follicular Microenvironment
by
Huihua Wu, Rui Zhu, Bo Zheng, Guizhi Liao, Fuxin Wang, Jie Ding, Hong Li and Mingqing Li
Cited by 8 | Viewed by 4329
Abstract
The follicular microenvironment, including intra-follicular granulosa cells (GCs), is responsible for oocyte maturation and subsequent ovulation. However, the functions of GCs and cellular components of the follicular microenvironment in preovulatory follicles have not been extensively explored. Here, we surveyed the single-cell transcriptome of
[...] Read more.
The follicular microenvironment, including intra-follicular granulosa cells (GCs), is responsible for oocyte maturation and subsequent ovulation. However, the functions of GCs and cellular components of the follicular microenvironment in preovulatory follicles have not been extensively explored. Here, we surveyed the single-cell transcriptome of the follicular microenvironment around MII oocytes in six human preovulatory follicles in in vitro fertilization. There were six different cell types in the preovulatory follicles, including GCs and various immune cells. In GCs, we identified nine different functional clusters with different functional transcriptomic profiles, including specific clusters involved in inflammatory responses and adhesive function. Follicular macrophages are involved in immune responses, extracellular matrix remoulding and assist GCs in promoting the oocyte meiotic resumption. Interestingly, we observed that the specific terminal state subcluster of GCs with high levels of adhesive-related molecules should result in macrophage recruitment and residence, further contributing to an obvious heterogeneity of the immune cell proportion in preovulatory follicles from different patients. Our results provide a comprehensive understanding of the transcriptomic landscape of the preovulatory follicular microenvironment at the single-cell level. It provides valuable insights into understanding the regulation of the oocyte maturation and ovulation process, offering potential clues for the diagnosis and treatment of oocyte-maturation-related and ovulation-related diseases.
Full article
►▼
Show Figures
Open AccessReview
Insight on Polyunsaturated Fatty Acids in Endometrial Receptivity
by
Min Chen, Zimeng Zheng, Jialu Shi and Jun Shao
Cited by 7 | Viewed by 3227
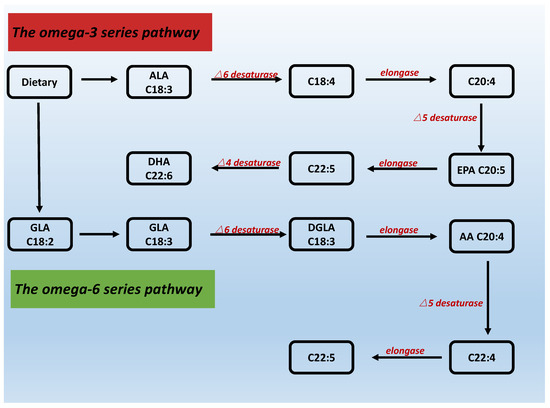
Abstract
Endometrial receptivity plays a crucial role in fertilization as well as pregnancy outcome in patients faced with fertility challenges. The optimization of endometrial receptivity may help with normal implantation of the embryo, and endometrial receptivity may be affected by numerous factors. Recently, the
[...] Read more.
Endometrial receptivity plays a crucial role in fertilization as well as pregnancy outcome in patients faced with fertility challenges. The optimization of endometrial receptivity may help with normal implantation of the embryo, and endometrial receptivity may be affected by numerous factors. Recently, the role of lipids in pregnancy has been increasingly recognized. Fatty acids and their metabolites may be involved in all stages of pregnancy and play a role in supporting cell proliferation and development, participating in cell signaling and regulating cell function. Polyunsaturated fatty acids, in particular, are essential fatty acids for the human body that can affect the receptivity of the endometrium through in a variety of methods, such as producing prostaglandins, estrogen and progesterone, among others. Additionally, polyunsaturated fatty acids are also involved in immunity and the regulation of endometrial decidualization. Fatty acids are essential for fetal placental growth and development. The interrelationship of polyunsaturated fatty acids with these substances and how they may affect endometrial receptivity will be reviewed in this article.
Full article
►▼
Show Figures
Planned Papers
The below list represents only planned manuscripts. Some of these
manuscripts have not been received by the Editorial Office yet. Papers
submitted to MDPI journals are subject to peer-review.
Title: To be determined
Authors: Sandra E. Reznik; et al.
Affiliation: College of Pharmacy and Health Sciences, St. John’s University, Queens, NY 11439, USA
Title: To be determined
Authors: Maurice-Andre Recanati; et al.
Affiliation: Department of Obstetrics and Gynecology, Wayne State University, Detroit, MI 48201, USA
Title: To be determined
Authors: Katsueki Ogiwara; et al.
Affiliation: Laboratory of Reproductive and Developmental Biology, Faculty of Science, Hokkaido University, Sapporo, Japan