Mesenchymal Stem Cell Fate and Potential Therapy
Share This Topical Collection
Editor
 Prof. Dr. Dafna Benayahu
Prof. Dr. Dafna Benayahu
 Prof. Dr. Dafna Benayahu
Prof. Dr. Dafna Benayahu
E-Mail
Website
Collection Editor
Department of Cell and Developmental Biology, Sackler Faculty of Medicine, Tel Aviv University, Ramat Aviv, Tel Aviv 6997801, Israel
Interests: marine bio-resources; stem cells differentiation; cells niche; scaffold biomaterials
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
The study of stem cells within mesenchymal systems relates to cells derived from the bone and bone marrow, cartilage, muscle, and connective and adipose tissue. Stems are rare, and they allow the production of precursor cells in adult life that also maintains self-renewal and multilineage differentiation. A small population of multipotent cells has the capacity for their self-renewing and clonogenic capacity to generate progeny cells that are differentiated into various lineages.
Knowing how to identify the stem/progenitor cells to characterize and separate them can allow maintaining them into distinct subpopulations based on both phenotype and function. Stem cell differentiation relies on transcription factors and chromatin remodeling that play a role in the cells’ regulation. The regulation of cell fate decisions is profoundly influenced by systemic and local signals that provide the cells’ niche. Determining the cells’ fate is required both to maintain tissue homeostasis and function in tissue regeneration. The use of progenitor cells with scaffold biomaterial makes them an enormous power for medical devices in a variety of therapeutic applications.
Prof. Dr. Dafna Benayahu
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Biomolecules is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs).
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Keywords
- mesenchymal stem cell fate
- stem/progenitors cells characterization
- stem cell differentiation
- cells niche
- scaffold biomaterial
Published Papers (17 papers)
Open AccessReview
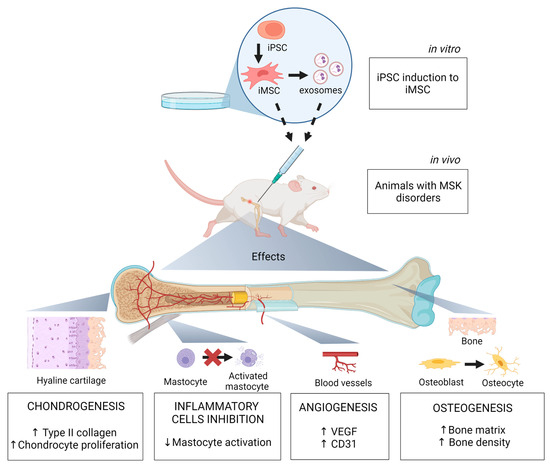
Potential and Limitations of Induced Pluripotent Stem Cells-Derived Mesenchymal Stem Cells in Musculoskeletal Disorders Treatment
by
Isabelle Xavier Dias, Aline Cordeiro, João Antonio Matheus Guimarães and Karina Ribeiro Silva
Cited by 1 | Viewed by 2271
Abstract
The burden of musculoskeletal disorders (MSK) is increasing worldwide. It affects millions of people worldwide, decreases their quality of life, and can cause mortality. The treatment of such conditions is challenging and often requires surgery. Thus, it is necessary to discuss new strategies.
[...] Read more.
The burden of musculoskeletal disorders (MSK) is increasing worldwide. It affects millions of people worldwide, decreases their quality of life, and can cause mortality. The treatment of such conditions is challenging and often requires surgery. Thus, it is necessary to discuss new strategies. The therapeutic potential of mesenchymal stem cells (MSC) in several diseases has been investigated with relative success. However, this potential is hindered by their limited stemness and expansion ability in vitro and their high donor variability. MSC derived from induced pluripotent stem cells (iPSC) have emerged as an alternative treatment for MSK diseases. These cells present distinct features, such as a juvenile phenotype, in addition to higher stemness, proliferation, and differentiation potential than those of MSC. Here, we review the opportunities, challenges, and applications of iPSC as relevant clinical therapeutic cell sources for MSK disorders. We discuss iPSC sources from which to derive iMSC and the advantages and disadvantages of iMSC over MSC as a therapeutic approach. We further summarize the main preclinical and clinical studies exploring the therapeutic potential of iMSC in MSK disorders.
Full article
►▼
Show Figures
Open AccessArticle
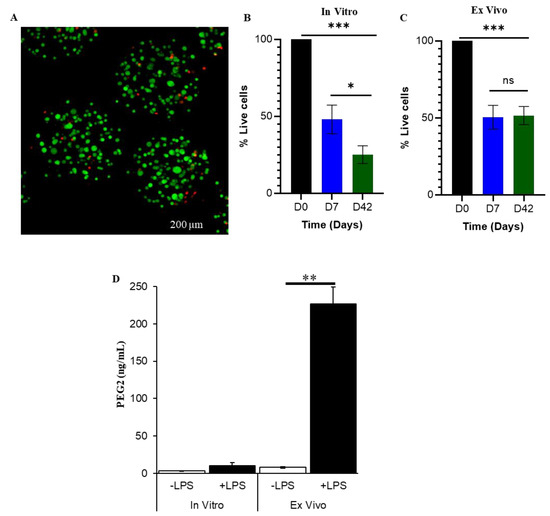
Anti-Inflammatory Effects of Encapsulated Human Mesenchymal Stromal/Stem Cells and a Method to Scale-Up Cell Encapsulation
by
Suneel Kumar, Maciej Kabat, Sayantani Basak, Joanne Babiarz, Francois Berthiaume and Martin Grumet
Cited by 4 | Viewed by 1599
Abstract
Mesenchymal stem/stromal cells (MSC) promote recovery in a wide range of animal models of injury and disease. They can act in vivo by differentiating and integrating into tissues, secreting factors that promote cell growth and control inflammation, and interacting directly with host effector
[...] Read more.
Mesenchymal stem/stromal cells (MSC) promote recovery in a wide range of animal models of injury and disease. They can act in vivo by differentiating and integrating into tissues, secreting factors that promote cell growth and control inflammation, and interacting directly with host effector cells. We focus here on MSC secreted factors by encapsulating the cells in alginate microspheres, which restrict cells from migrating out while allowing diffusion of factors including cytokines across the capsules. One week after intrathecal lumbar injection of human bone marrow MSC encapsulated in alginate (eMSC), rat IL-10 expression was upregulated in distant rat spinal cord injury sites. Detection of human IL-10 protein in rostrally derived cerebrospinal fluid (CSF) indicated distribution of this human MSC-secreted cytokine throughout rat spinal cord CSF. Intraperitoneal (IP) injection of eMSC in a rat model for endotoxemia reduced serum levels of inflammatory cytokines within 5 h. Detection of human IL-6 in sera after injection of human eMSC indicates rapid systemic distribution of this human MSC-secreted cytokine. Despite proof of concept for eMSC in various disorders using animal models, translation of encapsulation technology has not been feasible primarily because methods for scale-up are not available. To scale-up production of eMSC, we developed a rapid, semi-continuous, capsule collection system coupled to an electrosprayer. This system can produce doses of encapsulated cells sufficient for use in clinical translation.
Full article
►▼
Show Figures
Open AccessArticle
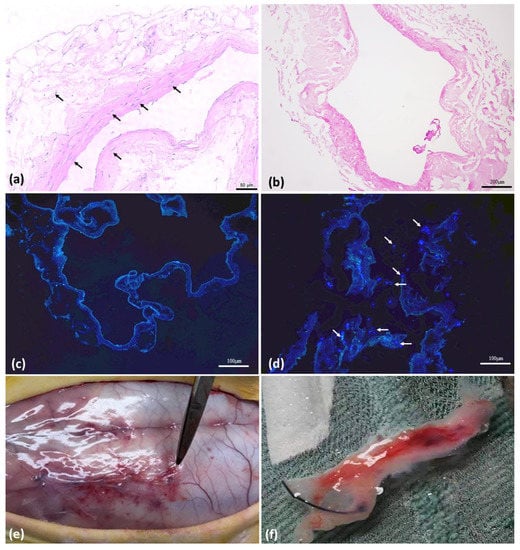
Evaluation of Biointegration and Inflammatory Response to Blood Vessels Produced by Tissue Engineering—Experimental Model in Rabbits
by
Mariana Thaís Silva Secondo, Lenize da Silva Rodrigues, Leandro Pereira Miranda Ramos, Ana Lívia Carvalho Bovolato, Diego Noé Rodriguez-Sanchez, Marcone Lima Sobreira, Marcelo Padovani de Toledo Moraes and Matheus Bertanha
Viewed by 1236
Abstract
Peripheral arterial disease (PAD) is the main cause of mortality in the western population and requires surgical intervention with the use of vascular substitutes, such as autologous veins or Dacron or PTFE prostheses. When this is not possible, it progresses to limb amputation.
[...] Read more.
Peripheral arterial disease (PAD) is the main cause of mortality in the western population and requires surgical intervention with the use of vascular substitutes, such as autologous veins or Dacron or PTFE prostheses. When this is not possible, it progresses to limb amputation. For cases where there is no autologous vascular substitute, tissue engineering with the production of neovessels may be a promising option. Previous experimental studies have shown in vitro that rabbit vena cava can be decellularized and serve as a scaffold for receiving mesenchymal stem cells (MSC), with subsequent differentiation into endothelial cells. The current study aimed to evaluate the behavior of a 3D product structure based on decellularized rabbit inferior vena cava (IVC) scaffolds seeded with adipose-tissue-derived stem cells (ASCs) and implanted in rabbits dorsally subcutaneously. We evaluated the induction of the inflammatory response in the animal. We found that stem cells were positive in reducing the inflammatory response induced by the decellularized scaffolds.
Full article
►▼
Show Figures
Open AccessFeature PaperArticle
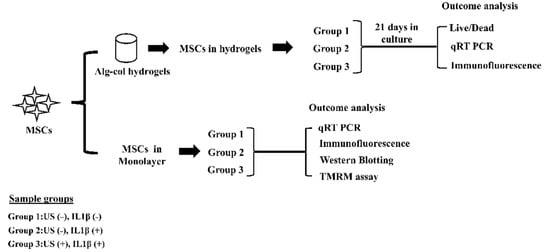
Continuous Low-Intensity Ultrasound Preserves Chondrogenesis of Mesenchymal Stromal Cells in the Presence of Cytokines by Inhibiting NFκB Activation
by
Sarayu Bhogoju, Shahid Khan and Anuradha Subramanian
Cited by 2 | Viewed by 2041
Abstract
Proinflammatory joint environment, coupled with impeded chondrogenic differentiation of mesenchymal stromal cells (MSCs), led to inferior cartilage repair outcomes. Nuclear translocation of phosphorylated-NFκB downregulates SOX9 and hinders the chondrogenesis of MSCs. Strategies that minimize the deleterious effects of NFκB, while promoting MSC chondrogenesis,
[...] Read more.
Proinflammatory joint environment, coupled with impeded chondrogenic differentiation of mesenchymal stromal cells (MSCs), led to inferior cartilage repair outcomes. Nuclear translocation of phosphorylated-NFκB downregulates SOX9 and hinders the chondrogenesis of MSCs. Strategies that minimize the deleterious effects of NFκB, while promoting MSC chondrogenesis, are of interest. This study establishes the ability of continuous low-intensity ultrasound (cLIUS) to preserve MSC chondrogenesis in a proinflammatory environment. MSCs were seeded in alginate:collagen hydrogels and cultured for 21 days in an ultrasound-assisted bioreactor (5.0 MHz, 2.5 Vpp; 4 applications/day) in the presence of IL1β and evaluated by qRT-PCR and immunofluorescence. The differential expression of markers associated with the NFκB pathway was assessed upon a single exposure of cLIUS and assayed by Western blotting, qRT-PCR, and immunofluorescence. Mitochondrial potential was evaluated by tetramethylrhodamine methyl ester (TMRM) assay. The chondroinductive potential of cLIUS was noted by the increased expression of SOX9 and COLII. cLIUS extended its chondroprotective effects by stabilizing the NFκB complex in the cytoplasm via engaging the IκBα feedback mechanism, thus preventing its nuclear translocation. cLIUS acted as a mitochondrial protective agent by restoring the mitochondrial potential and the mitochondrial mRNA expression in a proinflammatory environment. Altogether, our results demonstrated the potential of cLIUS for cartilage repair and regeneration under proinflammatory conditions.
Full article
►▼
Show Figures
Open AccessEditor’s ChoiceReview
Potential of Using Infrapatellar–Fat–Pad–Derived Mesenchymal Stem Cells for Therapy in Degenerative Arthritis: Chondrogenesis, Exosomes, and Transcription Regulation
by
Hsiu-Jung Liao, Chih-Hung Chang, Chi-Ying F. Huang and Hui-Ting Chen
Cited by 7 | Viewed by 3909
Abstract
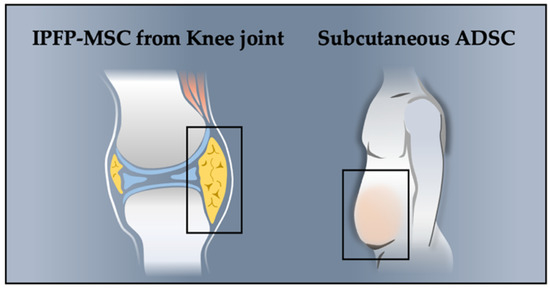
Infrapatellar fat pad–derived mesenchymal stem cells (IPFP-MSCs) are a type of adipose-derived stem cell (ADSC). They potentially contribute to cartilage regeneration and modulation of the immune microenvironment in patients with osteoarthritis (OA). The ability of IPFP-MSCs to increase chondrogenic capacity has been reported
[...] Read more.
Infrapatellar fat pad–derived mesenchymal stem cells (IPFP-MSCs) are a type of adipose-derived stem cell (ADSC). They potentially contribute to cartilage regeneration and modulation of the immune microenvironment in patients with osteoarthritis (OA). The ability of IPFP-MSCs to increase chondrogenic capacity has been reported to be greater, less age dependent, and less affected by inflammatory changes than that of other MSCs. Transcription-regulatory factors strictly regulate the cartilage differentiation of MSCs. However, few studies have explored the effect of transcriptional factors on IPFP-MSC-based neocartilage formation, cartilage engineering, and tissue functionality during and after chondrogenesis. Instead of intact MSCs, MSC-derived extracellular vesicles could be used for the treatment of OA. Furthermore, exosomes are increasingly being considered the principal therapeutic agent in MSC secretions that is responsible for the regenerative and immunomodulatory functions of MSCs in cartilage repair. The present study provides an overview of advancements in enhancement strategies for IPFP-MSC chondrogenic differentiation, including the effects of transcriptional factors, the modulation of released exosomes, delivery mechanisms for MSCs, and ethical and regulatory points concerning the development of MSC products. This review will contribute to the understanding of the IPFP-MSC chondrogenic differentiation process and enable the improvement of IPFP-MSC-based cartilage tissue engineering.
Full article
►▼
Show Figures
Open AccessArticle
Exploring the Cell Stemness and the Complexity of the Adipose Tissue Niche
by
Nadav Kislev, Roza Izgilov, Raizel Adler and Dafna Benayahu
Cited by 8 | Viewed by 3117
Abstract
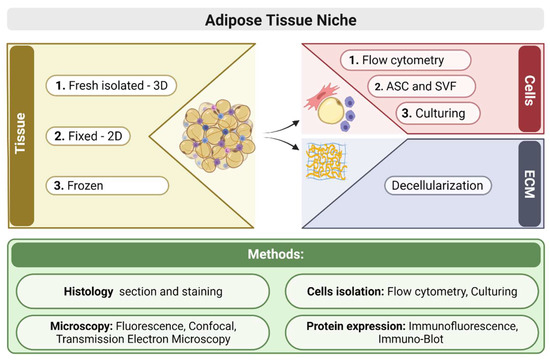
Adipose tissue is a complex organ composed of different cellular populations, including mesenchymal stem and progenitor cells, adipocytes, and immune cells such as macrophages and lymphocytes. These cellular populations alter dynamically during aging or as a response to pathophysiology such as obesity. Changes
[...] Read more.
Adipose tissue is a complex organ composed of different cellular populations, including mesenchymal stem and progenitor cells, adipocytes, and immune cells such as macrophages and lymphocytes. These cellular populations alter dynamically during aging or as a response to pathophysiology such as obesity. Changes in the various inflammatory cells are associated with metabolic complications and the development of insulin resistance, indicating that immune cells crosstalk with the adipocytes. Therefore, a study of the cell populations in the adipose tissue and the extracellular matrix maintaining the tissue niche is important for the knowledge on the regulatory state of the organ. We used a combination of methods to study various parameters to identify the composition of the resident cells in the adipose tissue and evaluate their profile. We analyzed the tissue structure and cells based on histology, immune fluorescence staining, and flow cytometry of cells present in the tissue in vivo and these markers’ expression in vitro. Any shift in cells’ composition influences self-renewal of the mesenchymal progenitors, and other cells affect the functionality of adipogenesis.
Full article
►▼
Show Figures
Open AccessArticle
Atelocollagen-Embedded Chondrocyte Precursors as a Treatment for Grade-4 Cartilage Defects of the Femoral Condyle: A Case Series with up to 9-Year Follow-Up
by
Hwa-Chang Liu, Tzu-Shang Thomas Liu, Yen-Liang Liu, Jyh-Horng Wang, Chih-Hung Chang, Tiffany Ting-Fang Shih and Feng-Huei Lin
Cited by 5 | Viewed by 3207
Abstract
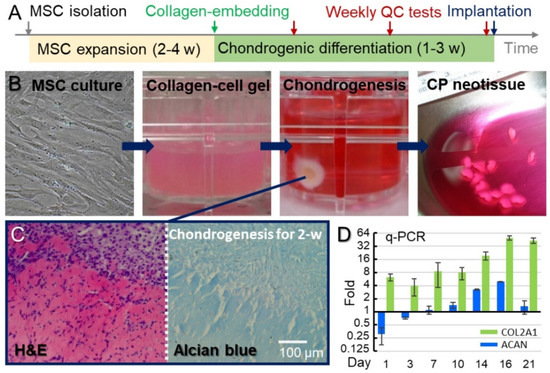
We demonstrated the safety and efficacy of autologous chondrocyte precursor (CP) cell therapy in repairing Grade 4 cartilage defects of medial femoral condyles. The autologous bone marrow mesenchymal stem cells of each participant were isolated, amplified, and then differentiated into CPs in atelocollagen.
[...] Read more.
We demonstrated the safety and efficacy of autologous chondrocyte precursor (CP) cell therapy in repairing Grade 4 cartilage defects of medial femoral condyles. The autologous bone marrow mesenchymal stem cells of each participant were isolated, amplified, and then differentiated into CPs in atelocollagen. Neotissues made of CPs were implanted into cartilage defects with an average cell density of 4.9 ± 2.1 × 10
6 cells/cm
2 through arthrotomy. The knee function was evaluated with the International Knee Documentation Committee (IKDC) subjective knee form. Patients’ knee functions significantly improved by the 28th week (IKDC score = 68.3 ± 12.1), relative to the initial functionality before the CP therapy (IKDC score = 46.1 ± 16.4,
p-value = 0.0014). Nine of these twelve patients maintained good knee functions for 9 years post-implantation (IKDC score = 69.8 ± 12.3) at levels higher than the pre-implantation values (
p-value = 0.0018). Patients were evaluated with MRI and arthroscopy, and the defective sites exhibited a smooth surface without a gap between the implant and host tissue. This study demonstrates that autologous CPs successfully engraft into the host tissue and result in the re-formation of hyaline-like cartilage, thereby improving the impaired knee functions. Most importantly, no adverse event was reported during this long-term follow-up period.
Full article
►▼
Show Figures
Open AccessArticle
FITC-Dextran Release from Cell-Embedded Fibrin Hydrogels
by
Viki Raz Lepsky, Sari Natan, Oren Tchaicheeyan, Avraham Kolel, Merav Zussman, Meital Zilberman and Ayelet Lesman
Cited by 6 | Viewed by 4325
Abstract
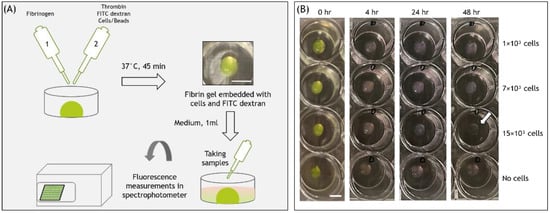
Fibrin hydrogel is a central biological material in tissue engineering and drug delivery applications. As such, fibrin is typically combined with cells and biomolecules targeted to the regenerated tissue. Previous studies have analyzed the release of different molecules from fibrin hydrogels; however, the
[...] Read more.
Fibrin hydrogel is a central biological material in tissue engineering and drug delivery applications. As such, fibrin is typically combined with cells and biomolecules targeted to the regenerated tissue. Previous studies have analyzed the release of different molecules from fibrin hydrogels; however, the effect of embedded cells on the release profile has yet to be quantitatively explored. This study focused on the release of Fluorescein isothiocyanate (FITC)-dextran (FD) 250 kDa from fibrin hydrogels, populated with different concentrations of fibroblast or endothelial cells, during a 48-h observation period. The addition of cells to fibrin gels decreased the overall release by a small percentage (by 7–15% for fibroblasts and 6–8% for endothelial cells) relative to acellular gels. The release profile was shown to be modulated by various cellular activities, including gel degradation and physical obstruction to diffusion. Cell-generated forces and matrix deformation (i.e., densification and fiber alignment) were not found to significantly influence the release profiles. This knowledge is expected to improve fibrin integration in tissue engineering and drug delivery applications by enabling predictions and ways to modulate the release profiles of various biomolecules.
Full article
►▼
Show Figures
Open AccessEditor’s ChoiceArticle
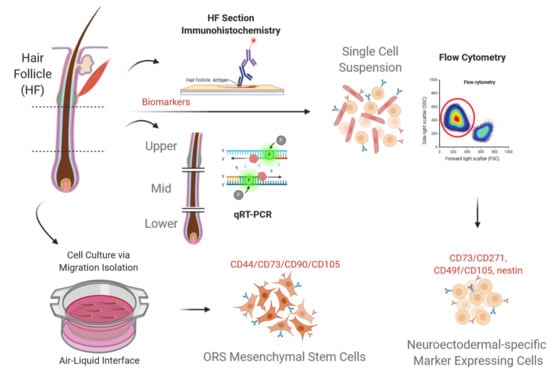
The Middle Part of the Plucked Hair Follicle Outer Root Sheath Is Identified as an Area Rich in Lineage-Specific Stem Cell Markers
by
Hanluo Li, Federica Francesca Masieri, Marie Schneider, Alexander Bartella, Sebastian Gaus, Sebastian Hahnel, Rüdiger Zimmerer, Ulrich Sack, Danijela Maksimovic-Ivanic, Sanja Mijatovic, Jan-Christoph Simon, Bernd Lethaus and Vuk Savkovic
Cited by 10 | Viewed by 5553
Abstract
Hair follicle outer root sheath (ORS) is a putative source of stem cells with therapeutic capacity. ORS contains several multipotent stem cell populations, primarily in the distal compartment of the bulge region. However, the bulge is routinely obtained using invasive isolation methods, which
[...] Read more.
Hair follicle outer root sheath (ORS) is a putative source of stem cells with therapeutic capacity. ORS contains several multipotent stem cell populations, primarily in the distal compartment of the bulge region. However, the bulge is routinely obtained using invasive isolation methods, which require human scalp tissue ex vivo. Non-invasive sampling has been standardized by means of the plucking procedure, enabling to reproducibly obtain the mid-ORS part. The mid-ORS shows potential for giving rise to multiple stem cell populations in vitro. To demonstrate the phenotypic features of distal, middle, and proximal ORS parts, gene and protein expression profiles were studied in physically separated portions. The mid-part of the ORS showed a comparable or higher NGFR, nestin/
NES, CD34,
CD73,
CD44,
CD133,
CK5,
PAX3,
MITF, and PMEL expression on both protein and gene levels, when compared to the distal ORS part. Distinct subpopulations of cells exhibiting small and round morphology were characterized with flow cytometry as simultaneously expressing CD73/CD271, CD49f/CD105, nestin, and not CK10. Potentially, these distinct subpopulations can give rise to cultured neuroectodermal and mesenchymal stem cell populations in vitro. In conclusion, the mid part of the ORS holds the potential for yielding multiple stem cells, in particular mesenchymal stem cells.
Full article
►▼
Show Figures
Open AccessArticle
Kartogenin Enhances Chondrogenic Differentiation of MSCs in 3D Tri-Copolymer Scaffolds and the Self-Designed Bioreactor System
by
Ching-Yun Chen, Chunching Li, Cherng-Jyh Ke, Jui-Sheng Sun and Feng-Huei Lin
Cited by 11 | Viewed by 3136
Abstract
Human cartilage has relatively slow metabolism compared to other normal tissues. Cartilage damage is of great clinical consequence since cartilage has limited intrinsic healing potential. Cartilage tissue engineering is a rapidly emerging field that holds great promise for tissue function repair and artificial/engineered
[...] Read more.
Human cartilage has relatively slow metabolism compared to other normal tissues. Cartilage damage is of great clinical consequence since cartilage has limited intrinsic healing potential. Cartilage tissue engineering is a rapidly emerging field that holds great promise for tissue function repair and artificial/engineered tissue substitutes. However, current clinical therapies for cartilage repair are less than satisfactory and rarely recover full function or return the diseased tissue to its native healthy state. Kartogenin (KGN), a small molecule, can promote chondrocyte differentiation both in vitro and in vivo. The purpose of this research is to optimize the chondrogenic process in mesenchymal stem cell (MSC)-based chondrogenic constructs with KGN for potential use in cartilage tissue engineering. In this study, we demonstrate that KGN treatment can promote MSC condensation and cell cluster formation within a tri-copolymer scaffold. Expression of
Acan,
Sox9, and
Col2a1 was significantly up-regulated in three-dimensional (3D) culture conditions. The lacuna-like structure showed active deposition of type II collagen and aggrecan deposition. We expect these results will open new avenues for the use of small molecules in chondrogenic differentiation protocols in combination with scaffolds, which may yield better strategies for cartilage tissue engineering.
Full article
►▼
Show Figures
Open AccessFeature PaperEditor’s ChoiceReview
Efficacy and Safety of Mesenchymal Stem/Stromal Cell Therapy for Inflammatory Bowel Diseases: An Up-to-Date Systematic Review
by
Jeffrey Zheng-Hsien Ko, Sheeva Johnson and Maneesh Dave
Cited by 32 | Viewed by 4231
Abstract
Inflammatory bowel disease (IBD) is a chronic inflammatory disorder of the gut that can lead to severe gastrointestinal symptoms, malnutrition, and complications such as fistulas and cancer. Mesenchymal stem/stromal cells (MSCs) are being investigated as a novel therapy for IBD and have been
[...] Read more.
Inflammatory bowel disease (IBD) is a chronic inflammatory disorder of the gut that can lead to severe gastrointestinal symptoms, malnutrition, and complications such as fistulas and cancer. Mesenchymal stem/stromal cells (MSCs) are being investigated as a novel therapy for IBD and have been demonstrated to be safe and effective for perianal fistulizing Crohn’s disease (PFCD). This systematic review aims to present the most recent studies on the safety and efficacy of MSC therapy in IBD. A detailed search strategy of clinical trials on MSCs and IBD was performed on PubMed, with 32 studies selected for inclusion in this review. The newest studies on local MSC injection for PFCD continue to support long-term efficacy while maintaining a favorable safety profile. The evidence for systemic MSC infusion in luminal IBD remains mixed due to marked methodological heterogeneity and unclear safety profiles. Although further studies are needed to better establish the role of this novel treatment modality, MSCs are proving to be a very exciting addition to the limited therapies available for IBD.
Full article
Open AccessArticle
In Vitro Differentiation of Human Placenta-Derived Multipotent Cells into Schwann-Like Cells
by
Chung-Hau Juan, Mei-Hsiu Chen, Feng-Hui Lin, Chih-Shung Wong, Chih-Cheng Chien and Ming-Hong Chen
Cited by 5 | Viewed by 2291
Abstract
Human placenta-derived multipotent stem cells (PDMCs) resembling embryonic stem cells can differentiate into three germ layer cells, including ectodermal lineage cells, such as neurons, astrocytes, and oligodendrocytes. The favorable characteristics of noninvasive cell harvesting include fewer ethical, religious, and legal considerations as well
[...] Read more.
Human placenta-derived multipotent stem cells (PDMCs) resembling embryonic stem cells can differentiate into three germ layer cells, including ectodermal lineage cells, such as neurons, astrocytes, and oligodendrocytes. The favorable characteristics of noninvasive cell harvesting include fewer ethical, religious, and legal considerations as well as accessible and limitless supply. Thus, PDMCs are attractive for cell-based therapy. The Schwann cell (SC) is the most common cell type used for tissue engineering such as nerve regeneration. However, the differentiation potential of human PDMCs into SCs has not been demonstrated until now. In this study, we evaluated the potential of PDMCs to differentiate into SC-like cells in a differentiation medium. After induction, PDMCs not only exhibited typical SC spindle-shaped morphology but also expressed SC markers, including S100, GFAP, p75, MBP, and Sox 10, as revealed by immunocytochemistry. Moreover, a reverse transcription-quantitative polymerase chain reaction analysis revealed the elevated gene expression of S100, GFAP, p75, MBP, Sox-10, and Krox-20 after SC induction. A neuroblastoma cell line, SH-SY5Y, was cultured in the conditioned medium (CM) collected from PDMC-differentiated SCs. The growth rate of the SH-SY5Y increased in the CM, indicating the function of PDMC-induced SCs. In conclusion, human PDMCs can be differentiated into SC-like cells and thus are an attractive alternative to SCs for cell-based therapy in the future.
Full article
►▼
Show Figures
Open AccessReview
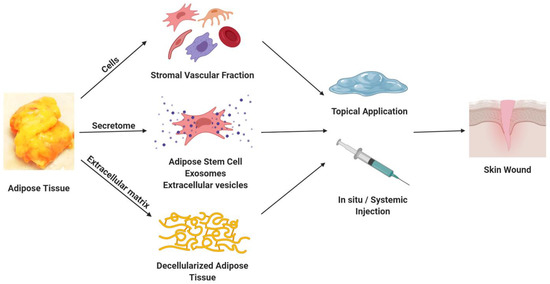
Clinical Translational Potential in Skin Wound Regeneration for Adipose-Derived, Blood-Derived, and Cellulose Materials: Cells, Exosomes, and Hydrogels
by
Trivia Frazier, Andrea Alarcon, Xiying Wu, Omair A. Mohiuddin, Jessica M. Motherwell, Anders H. Carlsson, Robert J. Christy, Judson V. Edwards, Robert T. Mackin, Nicolette Prevost, Elena Gloster, Qiang Zhang, Guangdi Wang, Daniel J. Hayes and Jeffrey M. Gimble
Cited by 26 | Viewed by 7284
Abstract
Acute and chronic skin wounds due to burns, pressure injuries, and trauma represent a substantial challenge to healthcare delivery with particular impacts on geriatric, paraplegic, and quadriplegic demographics worldwide. Nevertheless, the current standard of care relies extensively on preventive measures to mitigate pressure
[...] Read more.
Acute and chronic skin wounds due to burns, pressure injuries, and trauma represent a substantial challenge to healthcare delivery with particular impacts on geriatric, paraplegic, and quadriplegic demographics worldwide. Nevertheless, the current standard of care relies extensively on preventive measures to mitigate pressure injury, surgical debridement, skin flap procedures, and negative pressure wound vacuum measures. This article highlights the potential of adipose-, blood-, and cellulose-derived products (cells, decellularized matrices and scaffolds, and exosome and secretome factors) as a means to address this unmet medical need. The current status of this research area is evaluated and discussed in the context of promising avenues for future discovery.
Full article
►▼
Show Figures
Open AccessReview
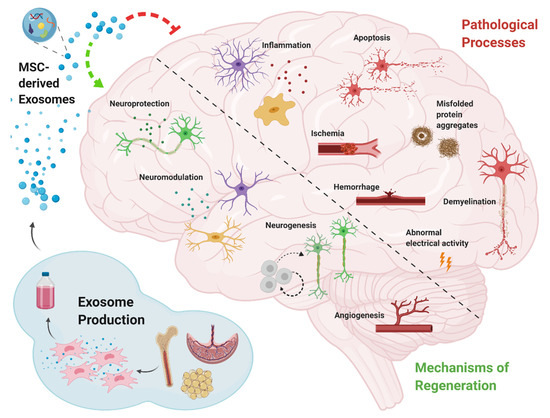
Promising Opportunities for Treating Neurodegenerative Diseases with Mesenchymal Stem Cell-Derived Exosomes
by
Reut Guy and Daniel Offen
Cited by 48 | Viewed by 5632
Abstract
Neurodegenerative disease refers to any pathological condition in which there is a progressive decline in neuronal function resulting from brain atrophy. Despite the immense efforts invested over recent decades in developing treatments for neurodegenerative diseases, effective therapy for these conditions is still an
[...] Read more.
Neurodegenerative disease refers to any pathological condition in which there is a progressive decline in neuronal function resulting from brain atrophy. Despite the immense efforts invested over recent decades in developing treatments for neurodegenerative diseases, effective therapy for these conditions is still an unmet need. One of the promising options for promoting brain recovery and regeneration is mesenchymal stem cell (MSC) transplantation. The therapeutic effect of MSCs is thought to be mediated by their secretome, and specifically, by their exosomes. Research shows that MSC-derived exosomes retain some of the characteristics of their parent MSCs, such as immune system modulation, regulation of neurite outgrowth, promotion of angiogenesis, and the ability to repair damaged tissue. Here, we summarize the functional outcomes observed in animal models of neurodegenerative diseases following MSC-derived exosome treatment. We will examine the proposed mechanisms of action through which MSC-derived exosomes mediate their therapeutic effects and review advanced studies that attempt to enhance the improvement achieved using MSC-derived exosome treatment, with a view towards future clinical use.
Full article
►▼
Show Figures
Open AccessReview
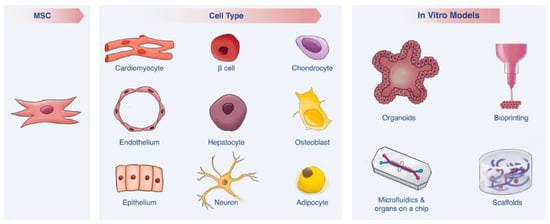
Mesenchymal Stem Cells as a Promising Cell Source for Integration in Novel In Vitro Models
by
Ann-Kristin Afflerbach, Mark D. Kiri, Tahir Detinis and Ben M. Maoz
Cited by 17 | Viewed by 6547
Abstract
The human-relevance of an in vitro model is dependent on two main factors—(i) an appropriate human cell source and (ii) a modeling platform that recapitulates human in vivo conditions. Recent years have brought substantial advancements in both these aspects. In particular, mesenchymal stem
[...] Read more.
The human-relevance of an in vitro model is dependent on two main factors—(i) an appropriate human cell source and (ii) a modeling platform that recapitulates human in vivo conditions. Recent years have brought substantial advancements in both these aspects. In particular, mesenchymal stem cells (MSCs) have emerged as a promising cell source, as these cells can differentiate into multiple cell types, yet do not raise the ethical and practical concerns associated with other types of stem cells. In turn, advanced bioengineered in vitro models such as microfluidics, Organs-on-a-Chip, scaffolds, bioprinting and organoids are bringing researchers ever closer to mimicking complex in vivo environments, thereby overcoming some of the limitations of traditional 2D cell cultures. This review covers each of these advancements separately and discusses how the integration of MSCs into novel in vitro platforms may contribute enormously to clinical and fundamental research.
Full article
►▼
Show Figures
Open AccessArticle
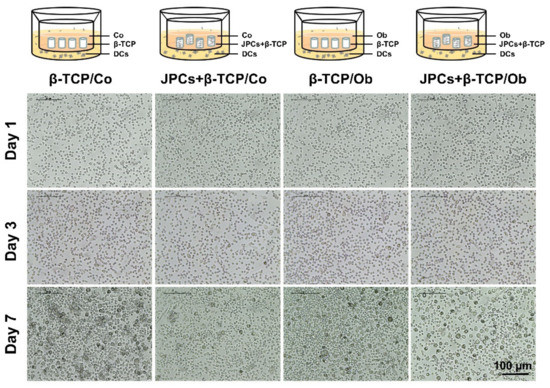
Jaw Periosteal Cells Seeded in Beta-Tricalcium Phosphate Inhibit Dendritic Cell Maturation
by
Jingtao Dai, Felix Umrath, Siegmar Reinert and Dorothea Alexander
Cited by 9 | Viewed by 2297
Abstract
Mesenchymal stem cells (MSCs) have gained attraction not only in the field of regenerative medicine but also in the field of autoimmune disease therapies or organ transplantation due to their immunoregulatory and/or immunosuppressive features. Dendritic cells (DCs) play a crucial role in initiating
[...] Read more.
Mesenchymal stem cells (MSCs) have gained attraction not only in the field of regenerative medicine but also in the field of autoimmune disease therapies or organ transplantation due to their immunoregulatory and/or immunosuppressive features. Dendritic cells (DCs) play a crucial role in initiating and regulating immune reactions by promoting antigen-specific T cell activation. In this study, we investigated the effect of human jaw periosteal progenitor cells (JPCs) seeded in beta-tricalcium phosphate (β-TCP) scaffolds on monocyte-derived DC differentiation. Significantly lower numbers of differentiated DCs were observed in the presence of normal (Co) and osteogenically induced (Ob) JPCs-seeded β-TCP constructs. Gene expression analysis revealed significantly lower interleukin-12 subunit p35 (IL-12p35) and interleukin-12 receptor beta 2 (IL-12Rβ2) and pro-inflammatory cytokine interferon-gamma (IFN-γ) levels in DCs under Ob conditions, while interleukin-8 (IL-8) gene levels were significantly increased. Furthermore, in the presence of JPCs-seeded β-TCP constructs, interleukin-10 (IL-10) gene expression was significantly induced in DCs, particularly under Ob conditions. Analysis of DC protein levels shows that granulocyte-colony stimulating factor (G-CSF) was significantly upregulated in coculture groups. Our results indicate that undifferentiated and osteogenically induced JPCs-seeded β-TCP constructs have an overall inhibitory effect on monocyte-derived DC maturation.
Full article
►▼
Show Figures
Open AccessArticle
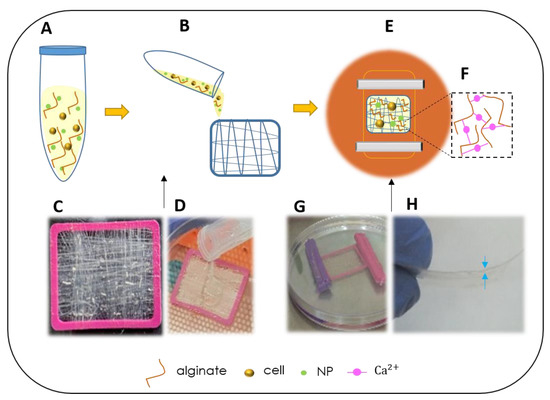
Mesenchymal Cell Growth and Differentiation on a New Biocomposite Material: A Promising Model for Regeneration Therapy
by
Leslie Pomeraniec and Dafna Benayahu
Cited by 12 | Viewed by 2511
Abstract
Mesenchymal stem cells serve as the body’s reservoir for healing and tissue regeneration. In cases of severe tissue trauma where there is also a need for tissue organization, a scaffold may be of use to support the cells in the damaged tissue. Such
[...] Read more.
Mesenchymal stem cells serve as the body’s reservoir for healing and tissue regeneration. In cases of severe tissue trauma where there is also a need for tissue organization, a scaffold may be of use to support the cells in the damaged tissue. Such a scaffold should be composed of a material that can biomimic the mechanical and biological properties of the target tissues in order to support autologous cell-adhesion, their proliferation, and differentiation. In this study, we developed and assayed a new biocomposite made of unique collagen fibers and alginate hydrogel that was assessed for the ability to support mesenchymal cell-proliferation and differentiation. Analysis over 11 weeks in vitro demonstrated that the scaffold was biocompatible and supports the cells viability and differentiation to produce tissue-like structures or become adipocyte under differentiation medium. When the biocomposite was enriched with nano particles (NPs), mesenchymal cells grew well after uptake of fluorescein isothiocyanate (FITC) labeled NPs, maintained their viability, migrated through the biocomposite, reached, and adhered to the tissue culture dish. These promising findings revealed that the scaffold supports the growth and differentiation of mesenchymal cells that demonstrate their full physiological function with no sign of material toxicity. The cells’ functionality performance indicates and suggests that the scaffold is suitable to be developed as a new medical device that has the potential to support regeneration and the production of functional tissue.
Full article
►▼
Show Figures