Feature Papers in 'Biomacromolecules: Proteins'
Share This Topical Collection
Editor
 Dr. Adrián Velázquez Campoy
Dr. Adrián Velázquez Campoy
 Dr. Adrián Velázquez Campoy
Dr. Adrián Velázquez Campoy
E-Mail
Website1
Website2
Collection Editor
1. Institute of Biocomputation and Physics of Complex Systems (BIFI), Universidad de Zaragoza, 50018 Zaragoza, Spain
2. Departamento de Bioquímica y Biología Molecular y Celular, Universidad de Zaragoza, 50009 Zaragoza, Spain
Interests: protein biophysics; protein interactions; protein stability and folding; cooperativity and allostery; biological calorimetry; drug discovery
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
This Topical Collection, “Feature Papers in Biomacromolecules: Proteins”, aims to collect high-quality research articles and comprehensive reviews on all aspects of proteins. It is dedicated to recent advances in the research area of proteins, and comprises a selection of exclusive papers from the Editorial Board Members (EBMs) of the Proteins Section, as well as invited papers from relevant experts. We also welcome senior experts in the field to make contributions to this Topical Collection. We aim to represent our Section as an attractive open-access publishing platform for biochemistry research.
Dr. Adrián Velázquez Campoy
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Biomolecules is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs).
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Published Papers (5 papers)
2023
Open AccessArticle
The Allosteric Regulation of Β-Ureidopropionase Depends on Fine-Tuned Stability of Active-Site Loops and Subunit Interfaces
by
Daniela Cederfelt, Dilip Badgujar, Ayan Au Musse, Bernhard Lohkamp, U. Helena Danielson and Doreen Dobritzsch
Viewed by 799
Abstract
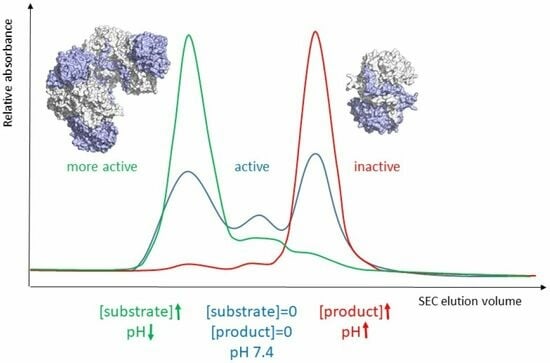
The activity of β-ureidopropionase, which catalyses the last step in the degradation of uracil, thymine, and analogous antimetabolites, is cooperatively regulated by the substrate and product of the reaction. This involves shifts in the equilibrium of the oligomeric states of the enzyme, but
[...] Read more.
The activity of β-ureidopropionase, which catalyses the last step in the degradation of uracil, thymine, and analogous antimetabolites, is cooperatively regulated by the substrate and product of the reaction. This involves shifts in the equilibrium of the oligomeric states of the enzyme, but how these are achieved and result in changes in enzyme catalytic competence has yet to be determined. Here, the regulation of human β-ureidopropionase was further explored via site-directed mutagenesis, inhibition studies, and cryo-electron microscopy. The active-site residue E207, as well as H173 and H307 located at the dimer–dimer interface, are shown to play crucial roles in enzyme activation. Dimer association to larger assemblies requires closure of active-site loops, which positions the catalytically crucial E207 stably in the active site. H173 and H307 likely respond to ligand-induced changes in their environment with changes in their protonation states, which fine-tunes the active-site loop stability and the strength of dimer–dimer interfaces and explains the previously observed pH influence on the oligomer equilibrium. The correlation between substrate analogue structure and effect on enzyme assembly suggests that the ability to favourably interact with F205 may distinguish activators from inhibitors. The cryo-EM structure of human β-ureidopropionase assembly obtained at low pH provides first insights into the architecture of its activated state. and validates our current model of the allosteric regulation mechanism. Closed entrance loop conformations and dimer–dimer interfaces are highly conserved between human and fruit fly enzymes.
Full article
►▼
Show Figures
Open AccessArticle
Structural and Functional Insights into the Stealth Protein CpsY of Mycobacterium tuberculosis
by
Dafeng Liu, Cai Yuan, Chenyun Guo, Mingdong Huang and Donghai Lin
Cited by 1 | Viewed by 1010
Abstract
Mycobacterium tuberculosis (
Mtb) is an important and harmful intracellular pathogen that is responsible for the cause of tuberculosis (TB).
Mtb capsular polysaccharides can misdirect the host’s immune response pathways, resulting in additional challenges in TB treatment. These capsule polysaccharides are biosynthesized
[...] Read more.
Mycobacterium tuberculosis (
Mtb) is an important and harmful intracellular pathogen that is responsible for the cause of tuberculosis (TB).
Mtb capsular polysaccharides can misdirect the host’s immune response pathways, resulting in additional challenges in TB treatment. These capsule polysaccharides are biosynthesized by stealth proteins, including CpsY. The structure and functional mechanism of
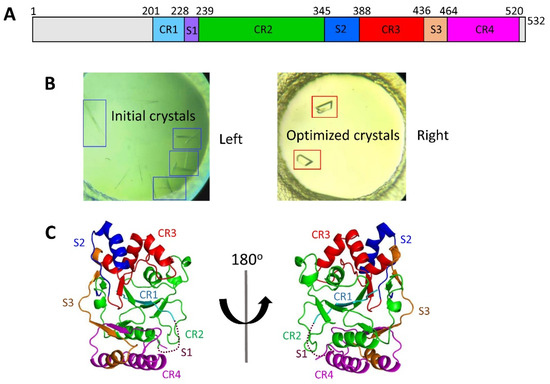
Mtb CpsY are not completely delineated. Here, we reported the crystal structure of CpsY
201−520 at 1.64 Å. CpsY
201−520 comprises three β-sheets with five α-helices on one side and three on the other. Four conserved regions (CR1–CR4) are located near and at the base of its catalytic cavity, and three spacer segments (S1–S3) surround the catalytic cavity. Site-directed mutagenesis demonstrated the strict conservation of R419 at CR3 and S1–S3 in regulating the phosphotransferase activity of CpsY
201−520. In addition, deletion of S2 or S3 (∆S2 or ∆S3) dramatically increased the activity compared to the wild-type (WT) CpsY
201−520. Results from molecular dynamics (MD) simulations showed that S2 and S3 are highly flexible. Our study provides new insights for the development of new vaccines and targeted immunotherapy against
Mtb.
Full article
►▼
Show Figures
Open AccessArticle
Biological Applications of Synthetic Binders Isolated from a Conceptually New Adhiron Library
by
Claudia D’Ercole, Matteo De March, Gianluca Veggiani, Sandra Oloketuyi, Rossella Svigelj and Ario de Marco
Viewed by 1465
Abstract
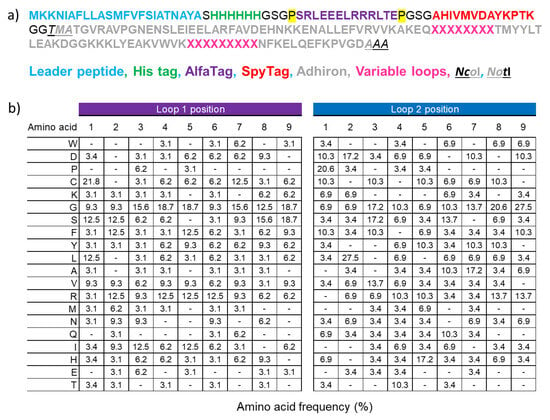
Background: Adhirons are small (10 kDa) synthetic ligands that might represent an alternative to antibody fragments and to alternative scaffolds such as DARPins or affibodies. Methods: We prepared a conceptionally new adhiron phage display library that allows the presence of cysteines in the
[...] Read more.
Background: Adhirons are small (10 kDa) synthetic ligands that might represent an alternative to antibody fragments and to alternative scaffolds such as DARPins or affibodies. Methods: We prepared a conceptionally new adhiron phage display library that allows the presence of cysteines in the hypervariable loops and successfully panned it against antigens possessing different characteristics. Results: We recovered binders specific for membrane epitopes of plant cells by panning the library directly against pea protoplasts and against soluble C-Reactive Protein and SpyCatcher, a small protein domain for which we failed to isolate binders using pre-immune nanobody libraries. The best binders had a binding constant in the low nM range, were produced easily in bacteria (average yields of 15 mg/L of culture) in combination with different tags, were stable, and had minimal aggregation propensity, independent of the presence or absence of cysteine residues in their loops. Discussion: The isolated adhirons were significantly stronger than those isolated previously from other libraries and as good as nanobodies recovered from a naïve library of comparable theoretical diversity. Moreover, they proved to be suitable reagents for ELISA, flow cytometry, the western blot, and also as capture elements in electrochemical biosensors.
Full article
►▼
Show Figures
Open AccessArticle
Newborns with Favourable Outcomes after Perinatal Asphyxia Have Upregulated Glucose Metabolism-Related Proteins in Plasma
by
Ping K. Yip, Michael Bremang, Ian Pike, Vennila Ponnusamy, Adina T. Michael-Titus and Divyen K. Shah
Viewed by 971
Abstract
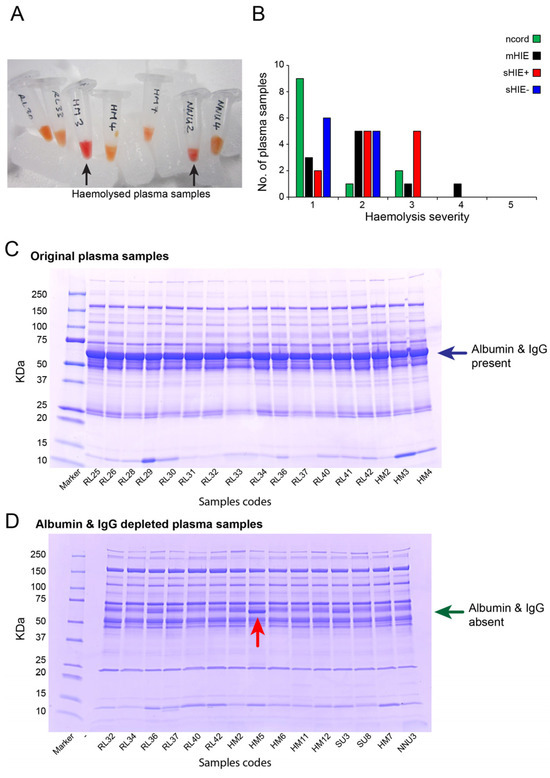
Hypoxic-ischaemic encephalopathy (HIE) is an important cause of morbidity and mortality globally. Although mild therapeutic hypothermia (TH) may improve outcomes in selected babies, the mechanism of action is not fully understood. A proteomics discovery study was carried out to analyse proteins in the
[...] Read more.
Hypoxic-ischaemic encephalopathy (HIE) is an important cause of morbidity and mortality globally. Although mild therapeutic hypothermia (TH) may improve outcomes in selected babies, the mechanism of action is not fully understood. A proteomics discovery study was carried out to analyse proteins in the plasma of newborns with HIE. Proteomic analysis of plasma from 22 newborns with moderate-severe HIE that had initially undergone TH, and relative controls including 10 newborns with mild HIE who did not warrant TH and also cord blood from 10 normal births (non-HIE) were carried out using the isobaric Tandem Mass Tag (TMT
®) 10plex
TM labelling with tandem mass spectrometry. A total of 7818 unique peptides were identified in all TMT10plex
TM samples, translating to 3457 peptides representing 405 proteins, after applying stringent filter criteria. Apart from the unique protein signature from normal cord blood, unsupervised analysis revealed several significantly regulated proteins in the TH-treated moderate-severe HIE group. GO annotation and functional clustering revealed various proteins associated with glucose metabolism: the enzymes fructose-bisphosphate aldolase A, glyceraldehyde-3-phosphate dehydrogenase, phosphoglycerate mutase 1, phosphoglycerate kinase 1, and pyruvate kinase PKM were upregulated in newborns with favourable (sHIE+) outcomes compared to newborns with unfavourable (sHIE−) outcomes. Those with favourable outcomes had normal MR imaging or mild abnormalities not predictive of adverse outcomes. However, in comparison to mild HIE and the sHIE− groups, the sHIE+ group had the additional glucose metabolism-related enzymes upregulated, including triosephosphate isomerase, α-enolase, 6-phosphogluconate dehydrogenase, transaldolase, and mitochondrial glutathione reductase. In conclusion, our plasma proteomic study demonstrates that TH-treated newborns with favourable outcomes have an upregulation in glucose metabolism. These findings may open new avenues for more effective neuroprotective therapy.
Full article
►▼
Show Figures
Open AccessFeature PaperArticle
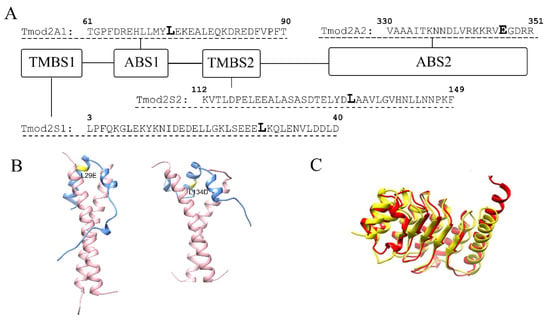
Effects of Tropomodulin 2 on Dendritic Spine Reorganization and Dynamics
by
Balaganesh Kuruba, Nickolas Starks, Mary Rose Josten, Ori Naveh, Gary Wayman, Marina Mikhaylova and Alla S. Kostyukova
Viewed by 1102
Abstract
Dendritic spines are actin-rich protrusions that receive a signal from the axon at the synapse. Remodeling of cytoskeletal actin is tightly connected to dendritic spine morphology-mediated synaptic plasticity of the neuron. Remodeling of cytoskeletal actin is required for the formation, development, maturation, and
[...] Read more.
Dendritic spines are actin-rich protrusions that receive a signal from the axon at the synapse. Remodeling of cytoskeletal actin is tightly connected to dendritic spine morphology-mediated synaptic plasticity of the neuron. Remodeling of cytoskeletal actin is required for the formation, development, maturation, and reorganization of dendritic spines. Actin filaments are highly dynamic structures with slow-growing/pointed and fast-growing/barbed ends. Very few studies have been conducted on the role of pointed-end binding proteins in the regulation of dendritic spine morphology. In this study, we evaluated the role played by tropomodulin 2 (Tmod2)—a brain-specific isoform, on the dendritic spine re-organization. Tmod2 regulates actin nucleation and polymerization by binding to the pointed end via actin and tropomyosin (Tpm) binding sites. We studied the effects of Tmod2 overexpression in primary hippocampal neurons on spine morphology using confocal microscopy and image analysis. Tmod2 overexpression decreased the spine number and increased spine length. Destroying Tpm-binding ability increased the number of shaft synapses and thin spine motility. Eliminating the actin-binding abilities of Tmod2 increased the number of mushroom spines. Tpm-mediated pointed-end binding decreased F-actin depolymerization, which may positively affect spine stabilization; the nucleation ability of Tmod2 appeared to increase shaft synapses.
Full article
►▼
Show Figures