Mitochondria and Stress Responses
A topical collection in Biology (ISSN 2079-7737). This collection belongs to the section "Cell Biology".
Viewed by 18130Editor
2.Ecole Pratique des Hautes Etudes, PSL Research University, 75014 Paris, France
Interests: apoptosis; cell death; mitochondria; cell stress; mitophagy; aging; drosophila
Topical Collection Information
Dear Colleagues,
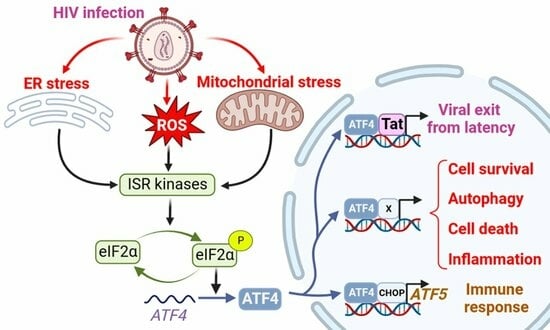
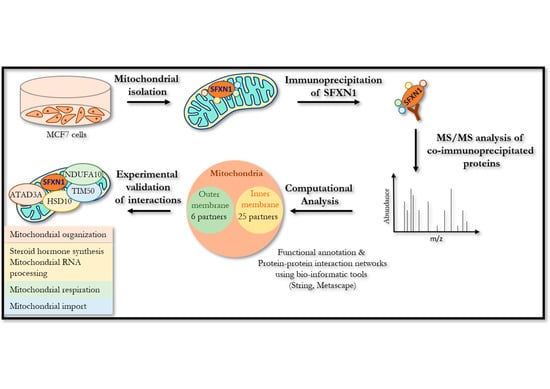
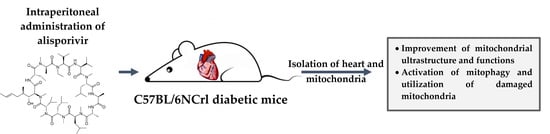
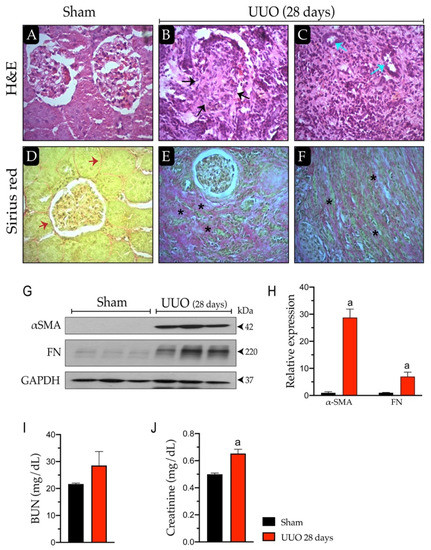
Mitochondria are generally known as the powerhouses of the cell. They play a central role in bioenergetics since they are the site of fatty acid β-oxidation, the tricarboxylic acid cycle, and oxidative phosphorylation. Mitochondria are also involved in various processes like pyrimidine synthesis, steroid hormone production, redox homeostasis, calcium storage, iron metabolism, etc. Mitochondria are involved in aging. They play a role in malignant tumor progression and respiratory chain dysfunctions, are responsible for multiple diseases referred to as mitochondriopathies, and defects in the mitochondrial network dynamics are associated with neurodegenerative disorders. According to this central position of mitochondria, various mechanisms involved in the quality control of the mitochondrial network have been selected during evolution. These mechanisms can operate at the protein level, using chaperones and proteases, at the organelle level (mitophagy), or at the cellular level (cell death). In addition to these responses to mitochondrial stress, mitochondria are also fully involved in the cell response to various stresses, such as DNA damage, viral infection, unbalanced proteostasis, and others. Notably, mitochondria are recognized today as central integrators of cell death and survival signals.
This Topical Collection entitled “Mitochondria and Stress Responses” aims to present articles focused on mitochondrial quality control mechanisms as well as on the role of mitochondria in cell stress responses. For example, articles dealing with subjects including but not restricted to mtUPR response, mitophagy, cell death processes involving mitochondria, and the crosstalk of mitochondria with other organelles are welcome, with an emphasis on recent developments in these fields. Original research and review articles are welcome.
Prof. Dr. Bernard Mignotte
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Biology is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- mitochondria
- cell death
- apoptosis
- cell stress
- mitophagy
- aging
- mitochondrial stress
- mtUPR
- mitochondrial quality control