Design, Synthesis and In Vitro Studies of 3-Amidocoumarins as Novel Antibiofilm Agents
Abstract
:1. Introduction
Inhibitor Design
2. Results and Discussion
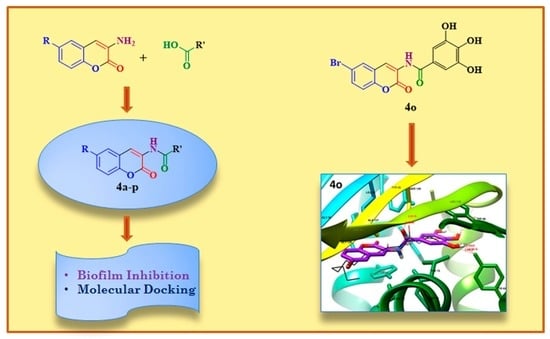
2.1. Chemistry
2.2. Biology
2.3. Docking Studies
3. Materials and Methods
3.1. Experimental
3.2. Chemistry
3.2.1. Preparation of 3-nitrocoumarin 1a and 6-bromo3-nitrocoumarin 1b
3.2.2. Preparation of 3-aminocoumarin 2a and 6-bromo-3-aminocoumarin 2b
3.2.3. General Procedure for the Synthesis of Compounds (4a–p)
4-Hydroxy-N-(2-oxo-2H-chromen-3-yl)benzamide (4a)
3-Chloro-N-(2-oxo-2H-chromen-3-yl)benzamide (4b)
2-Iodo-N-(2-oxo-2H-chromen-3-yl)benzamide (4c)
4-Nitro-N-(2-oxo-2H-chromen-3-yl)benzamide (4d)
2-Hydroxy-3,5-dinitro-N-(2-oxo-2H-chromen-3-yl)benzamide (4e)
N-(6-bromo-2-oxo-2H-chromen-3-yl)-4-hydroxybenzamide (4f)
N-(6-bromo-2-oxo-2H-chromen-3-yl)-3-chlorobenzamide (4g)
N-(6-bromo-2-oxo-2H-chromen-3-yl)-2-iodobenzamide (4h)
N-(6-bromo-2-oxo-2H-chromen-3-yl)-4-nitrobenzamide (4i)
N-(6-bromo-2-oxo-2H-chromen-3-yl)-2-hydroxy-3,5-dinitrobenzamide (4j)
N-(6-bromo-2-oxo-2H-chromen-3-yl)-2-methylbenzamide (4k)
N-(6-bromo-2-oxo-2H-chromen-3-yl)-2-hydroxybenzamide (4l)
5-Amino-N-(6-bromo-2-oxo-2H-chromen-3-yl)-2-hydroxybenzamide (4m)
N-(6-bromo-2-oxo-2H-chromen-3-yl)-2-hydroxy-3,5-diiodobenzamide (4n)
N-(6-bromo-2-oxo-2H-chromen-3-yl)-3,4,5-trihydroxybenzamide (4o)
N-(6-bromo-2-oxo-2H-chromen-3-yl)pyrrolidine-2-carboxamide (4p)
3.3. Biology
3.3.1. Evaluation of Antiquorum Sensing Activity of Compounds for Biofilm Inhibition
3.3.2. Biofilm Formation
3.3.3. Biofilm Inhibition Analysis
3.4. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial biofilm and its role in the pathogenesis of disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence 2018, 9, 522–554. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Hall, C.W.; Mah, T.-F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef]
- Rabin, N.; Zheng, Y.; Opoku-Temeng, C.; Du, Y.; Bonsu, E.; Sintim, H.O. Biofilm formation mechanisms and targets for developing antibiofilm agents. Future Med. Chem. 2015, 7, 493–512. [Google Scholar] [CrossRef]
- Koo, H.; Allan, R.N.; Howlin, R.P.; Stoodley, P.; Stoodley, L.H. Targeting microbial biofilms: Current and prospective therapeutic strategies. Nat. Rev. Microbiol. 2017, 15, 740–755. [Google Scholar] [CrossRef]
- Preda, V.G.; Săndulescu, O. Communication is the key: Biofilms, quorum sensing, formation and prevention. Discoveries 2019, 7, e100. [Google Scholar] [CrossRef]
- Sionov, R.V.; Steinberg, D. Targeting the holy triangle of quorum sensing, biofilm formation, and antibiotic resistance in pathogenic bacteria. Microorganisms 2022, 10, 1239. [Google Scholar] [CrossRef]
- Whiteley, M.; Diggle, S.; Greenberg, E. Progress in and promise of bacterial quorum sensing research. Nature 2017, 551, 313–320. [Google Scholar] [CrossRef]
- Miller, M.B.; Bassler, B.L. Quorum sensing in bacteria. Annu. Rev. Microbiol. 2001, 55, 165–199. [Google Scholar] [CrossRef]
- Rutherford, S.T.; Bassler, B.L. Bacterial quorum sensing: Its role in virulence and possibilities for its control. Cold Spring Harb. Perspect. Med. 2012, 2, a012427. [Google Scholar] [CrossRef]
- Ramanathan, S.; Sivasubramanian, S.; Pandurangan, P.; Mani, G.; Madhu, D.; Lin, X. Bacterial biofilm inhibition: A focused review on recent therapeutic strategies for combating the biofilm mediated infections. Front. Microbiol. 2021, 12, 676458. [Google Scholar] [CrossRef]
- Nadar, S.; Khan, T.; Patching, S.G.; Omri, A. Development of antibiofilm therapeutics strategies to overcome antimicrobial drug resistance. Microorganisms 2022, 10, 303. [Google Scholar] [CrossRef]
- Manefield, M.; de Nys, R.; Kumar, N.; Read, R.; Givskov, M.; Steinberg, P.; Kjelleberg, S.A. Evidence that halogenated furanones from Delisea pulchra inhibit acylated homoserine lactone (AHL)-mediated gene expression by displacing the AHL signal from its receptor protein. Microbiology 1999, 145, 283–291. [Google Scholar] [CrossRef]
- Park, J.S.; Ryu, E.-J.; Li, L.; Choi, B.-K.; Kim, B.M. New bicyclic brominated furanones as potent autoinducer-2 quorum-sensing inhibitors against bacterial biofilm formation. Eur. J. Med. Chem. 2017, 137, 76–87. [Google Scholar] [CrossRef]
- Yang, S.; Abdel-Razek, O.A.; Cheng, F.; Bandyopadhyay, D.; Shetye, G.S.; Wang, G.; Luk, Y.-Y. Bicyclic brominated furanones: A new class of quorum sensing modulators that inhibit bacterial biofilm formation. Bioorg. Med. Chem. 2014, 22, 1313–1317. [Google Scholar] [CrossRef]
- Almohaywi, B.; Yu, T.T.; Iskander, G.; Chan, D.S.H.; Ho, K.K.K.; Rice, S.; Black, D.S.; Griffith, R.; Kumar, N. Dihydropyrrolones as bacterial quorum sensing inhibitors. Bioorg. Med. Chem. Lett. 2019, 29, 1054–1059. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, P.; Qin, Y.; Zhang, N.; Teng, Y.; Venter, H.; Ma, S. Design and synthesis of aryl-substituted pyrrolidone derivatives as quorum sensing inhibitors. Bioorg. Chem. 2020, 105, 104376. [Google Scholar] [CrossRef]
- Nizalapur, S.; Kimyon, O.; Yee, E.; Bhadbhade, M.M.; Manefield, M.; Willcox, M.; Black, D.S.C.; Kumar, N. Synthesis and biological evaluation of novel acyclic and cyclic glyoxamide based derivatives as bacterial quorum sensing and biofilm inhibitors. Org. Biomol. Chem. 2017, 15, 5743–5755. [Google Scholar] [CrossRef]
- Singh, L.R.; Tripathi, V.C.; Raj, S.; Kumar, A.; Gupta, S.; Horam, S.; Upadhyay, A.; Kushwaha, P.; Arockiaraj, J.; Sashidhara, K.V.; et al. In-house chemical library repurposing: A case example for Pseudomonas aeruginosa antibiofilm activity and quorum sensing inhibition. Drug Dev. Res. 2018, 9, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Stowe, S.D.; Richards, J.J.; Tucker, A.T.; Thompson, R.; Melander, C.; Cavanagh, J. Anti-biofilm compounds derived from marine sponges. Mar. Drugs. 2011, 9, 2010–2035. [Google Scholar] [CrossRef] [PubMed]
- Herrera, K.M.S.; da Silva, F.K.; de Lima, W.G.; de Barbosa, S.C.; Gonçalves, A.M.M.N.; Viana, G.H.R.; Soares, A.C.; Ferreira, J.M.S. Antibacterial and antibiofilm activities of synthetic analogs of 3-alkylpyridine marine alkaloids. Med. Chem. Res. 2020, 29, 1084–1089. [Google Scholar] [CrossRef]
- El-Messery, S.M.; Habib, E.E.; Al-Rashood, S.T.A.; Hassan, G.S. Synthesis, antimicrobial, anti-biofilm evaluation, and molecular modelling study of new chalcone linked amines derivatives. J. Enzyme Inhib. Med. Chem. 2018, 33, 818–832. [Google Scholar] [CrossRef]
- Mairink, S.Z.; Barbosa, L.C.A.; Boukouvalas, J.; Pedroso, S.H.S.P.; Santos, S.G.; Magalhães, P.P.; Farias, L.M. Synthesis and evaluation of cadiolide analogues as inhibitors of bacterial biofilm formation. Med. Chem. Res. 2018, 27, 2426–2436. [Google Scholar] [CrossRef]
- Lal, J.; Kaul, G.; Akhir, A.; Ansari, S.B.; Chopra, S.; Reddy, D.N. Bio-evaluation of fluoro and trifluoromethyl-substituted salicylanilides against multidrug-resistant S. aureus. Med. Chem. Res. 2021, 30, 2301–2315. [Google Scholar] [CrossRef]
- Reen, F.J.; Gutiérrez-Barranquero, J.; Parages, M.L.; O’Gara, F. Coumarin: A novel player in microbial quorum sensing and biofilm formation inhibition. Appl. Microbiol. Biotechnol. 2018, 102, 2063–2073. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.G.; Cho, H.S.; Ryu, S.Y.; Cho, M.H.; Lee, J. Coumarins reduce biofilm formation and the virulence of Escherichia coli O157:H7. Phytomedicine 2014, 21, 1037–1042. [Google Scholar] [CrossRef]
- D’Almeida, R.E.; Molina, R.D.I.; Viola, C.M.; Luciardi, M.C.; Peñalver, C.N.; Bardón, A.; Arena, M.E. Comparison of seven structurally related coumarins on the inhibition of quorum sensing of Pseudomonas aeruginosa and Chromobacterium violaceum. Bioorg. Chem. 2017, 73, 37–42. [Google Scholar] [CrossRef]
- Ojima, Y.; Nunogami, S.; Taya, M. Antibiofilm effect of warfarin on biofilm formation of Escherichia coli promoted by antimicrobial treatment. J. Glob. Antimicrob. Resist. 2016, 7, 102–105. [Google Scholar] [CrossRef]
- Qais, F.A.; Khan, M.S.; Ahmad, I.; Husain, F.M.; Khan, R.A.; Hassan, I.; Shahzad, S.A.; AlHarbi, W. Coumarin exhibits broad-spectrum antibiofilm and antiquorum sensing activity against Gram-negative bacteria: In vitro and in silico investigation. ACS Omega 2021, 6, 18823–18835. [Google Scholar] [CrossRef]
- Sharma, R.K.; Singh, V.; Tiwari, N.; Butcher, R.J.; Katiyar, D. Synthesis, antimicrobial and chitinase inhibitory activities of 3-amidocoumarins. Bioorg. Chem. 2020, 98, 103700. [Google Scholar] [CrossRef]
- Shukla, M.; Singh, V.; Habeeballah, H.; Alkhanani, M.F.; Lata, M.; Hussain, Y.; Mukherjee, M.; Pasupuleti, M.; Meena, A.; Mishra, B.N.; et al. Quorum quenching guided inhibition of mixed bacterial biofilms and virulence properties by protein derived from leaves of Carissa carandas. Front. Cell. Infect. Microbiol. 2022, 12, 836819. [Google Scholar] [CrossRef]
- Sharma, R.K.; Priyanka; Katiyar, D. L-proline catalyzed condensation of salicylaldehydes with ethyl nitroacetate: An efficient access to 3-nitrocoumarins. Monatsh. Chem. 2016, 147, 2157–2161. [Google Scholar] [CrossRef]
- Hentzer, M.; Wu, H.; Andersen, J.B.; Riedel, K.; Rasmussen, T.B.; Bagge, N.; Kumar, N.; Schembri, M.A.; Song, Z.; Kristoffersen, P.; et al. Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J. 2003, 22, 3803–3815. [Google Scholar] [CrossRef]
- Bottomley, M.J.; Muraglia, E.; Bazzo, R.; Carfì, A. Molecular insights into quorum sensing in the human pathogen Pseudomonas aeruginosa from the structure of the virulence regulator LasR bound to its autoinducer. J. Biol. Chem. 2007, 282, 13592–13600. [Google Scholar] [CrossRef]
- Gavrilova, N.A.; Semichenko, E.S.; Korotchenko, O.S.; Suboch, G.A. Cyclocondensation of ethyl nitroacetate with 2-hydroxybenzaldehydes. Russ. J. Org. Chem. 2008, 44, 624–625. [Google Scholar] [CrossRef]
- Das, D.K.; Sarkar, S.; Khan, M.; Belal, M.; Khan, A.T. A mild and efficient method for large scale synthesis of 3-aminocoumarins and its further application for the preparation of 4-bromo-3-aminocoumarins. Tetrahedron Lett. 2014, 55, 4869–4874. [Google Scholar] [CrossRef]





| Synthesized Compounds | log P | Binding Energy | Compound | log P | Binding Energy | ||
|---|---|---|---|---|---|---|---|
| 4a |  | 1.72 | −11.36 | 4i |  | 3.62 | −10.52 |
| 4b |  | 2.67 | −10.51 | 4j |  | 3.20 | −10.31 |
| 4c |  | 3.47 | −10.32 | 4k |  | 3.43 | −11.25 |
| 4d |  | 2.74 | −9.84 | 4l |  | 2.55 | −11.66 |
| 4e |  | 2.31 | −10.46 | 4m |  | 1.75 | −11.31 |
| 4f |  | 2.55 | −11.47 | 4n |  | 5.26 | −9.44 |
| 4g |  | 3.50 | −10.68 | 4o |  | 1.77 | −11.69 |
| 4h |  | 4.30 | −11.65 | 4p |  | 0.95 | −11.03 |
| Entry | Binding Energy (kcal/mol) | Pose No. | Orientation * | H-Bond Interaction | Electrostatic Interaction | Hydrophobic and π Interactions |
|---|---|---|---|---|---|---|
| OdDHL (crystal str) | - | - | Trp60, Asp73, Tyr56, Ser129 | Tyr56, Tyr64, Asp73, Thr75, Ala105, Ser129 | Tyr47, Tyr64, Tyr93, Ala105, Leu110, Gly126 | |
| OdDHL (Docked) | −9.48 | 1 | Similar | Tyr56, Ser129, Tyr93, Thr75 | Trp88, Ala105, Phe101, Asp73, Leu110 | Leu36, Gly38, Leu39, Leu40, Tyr64, Leu125, Gly126, Ala127 |
| 4o | −11.69 | 7 | Flipped | Val111, Tyr93, Ser129, Tyr56, Arg61 | Leu36, Gly38, Trp88, Tyr64, Leu110, Asp73, Thr75, Ala105, Gly126, Ala127 | Leu36, Gly38, Ala127, Val76, Trp88, Tyr64 |
| 4l | −11.66 | 7 | Flipped | Ser129, Trp60, Arg61 | Leu36, Gly38, Tyr64, Tyr56, Asp73, Leu110, Gly126, Ala127 | Leu36, Gly38, Ile52, Tyr64, Thr75, Trp88, Tyr93, Ala105, Leu110 |
| 4h | −11.65 | 9 | Similar | Trp60, Arg61 | Tyr47, Leu36, Trp60, Arg61, Tyr56, Asp73, Trp88, Tyr93, Val76, Tyr64, Thr75 | Leu36, Leu39, Gly38, Ala50, Tyr56, Trp60, Tyr64, Gly126, Ala127 |
| 4f | −11.47 | 4 | Flipped | Leu110, Tyr93, Ser129, Tyr56, Arg61 | Leu36, Gly38, Leu39, Trp88, Tyr64, Asp73, Leu110, Leu125, Gly126, Ala127 | Leu36, Gly38, Ile52, Tyr64, Thr75, Trp88, Leu110 |
| 4a | −11.36 | 8 | Flipped | Tyr56, Arg6, Tyr93, Val111, Ser129 | Leu36, Tyr64, Trp88, Leu110, Asp73 | Leu36, Gly38, Trp88, Thr75, Ala127 |
| 4m | −11.31 | 7 | Flipped | Tyr93, Ser129, Trp60, Arg61 | Leu36, Gly38, Trp88, Tyr56, Tyr64, Asp73, Thr75, Leu110, Gly126, Ala127 | Leu36, Gly38, Tyr64, Trp88, Ala105, Leu110 |
| 4k | −11.25 | 10 | Flipped | Tyr56, Arg61, Ser129 | Leu36, Gly38, Leu39, Tyr64, Asp73, Leu125, Gly126, Ala127 | Leu36, Gly38, Ile52, Trp60, Tyr64, Thr75, Trp88, Tyr93, Leu110 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, R.K.; Singh, V.; Raghuvanshi, V.; Katiyar, D. Design, Synthesis and In Vitro Studies of 3-Amidocoumarins as Novel Antibiofilm Agents. Drugs Drug Candidates 2023, 2, 279-294. https://doi.org/10.3390/ddc2020015
Sharma RK, Singh V, Raghuvanshi V, Katiyar D. Design, Synthesis and In Vitro Studies of 3-Amidocoumarins as Novel Antibiofilm Agents. Drugs and Drug Candidates. 2023; 2(2):279-294. https://doi.org/10.3390/ddc2020015
Chicago/Turabian StyleSharma, Rajesh Kumar, Vineeta Singh, Vaishali Raghuvanshi, and Diksha Katiyar. 2023. "Design, Synthesis and In Vitro Studies of 3-Amidocoumarins as Novel Antibiofilm Agents" Drugs and Drug Candidates 2, no. 2: 279-294. https://doi.org/10.3390/ddc2020015