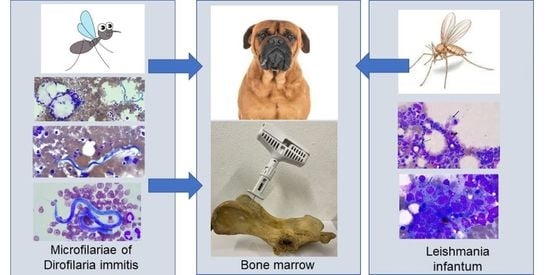
Incidental Finding of Dirofilaria immitis (Spirurida: Onchocercidae) Microfilariae in the Bone Marrow of a Dog with Mixed Leishmania infantum-Dirofilaria immitis Infection
Abstract
:Simple Summary
Abstract
1. Introduction
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pereira, A.; Maia, C. Leishmania infection in cats and feline leishmaniosis: An updated review with a proposal of a diagnosis algorithm and prevention guidelines. Curr. Res. Parasitol. Vector Borne Dis. 2021, 1, 100035. [Google Scholar] [CrossRef] [PubMed]
- Alcover, M.M.; Giner, J.; Rabasedas, J.; Roca-Geronés, X.; Verde, M.; Fernández, A.; Riera, C.; Fisa, R.; Villanueva-Saz, S. First epidemiological survey of Leishmania infantum in the domestic ferret (Mustela putorius furo) in a canine leishmaniosis endemic area using serology and PCR. Parasit. Vectors 2022, 15, 372. [Google Scholar] [CrossRef] [PubMed]
- Picard, M.; Soundaramourty, C.; Silvestre, R.; Estaquier, J.; André, S. Leishmania infantum infection of primary human myeloid cells. Microorganisms 2022, 10, 1243. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.H.; Wang, J.Y.; Zhang, S.; Yang, Y.T.; Wang, Y. Survey of wild and domestic mammals for infection with Leishmania infantum following an outbreak of desert zoonotic visceral leishmaniasis in Jiashi, People’s Republic of China. PLoS ONE 2015, 10, e0132493. [Google Scholar] [CrossRef]
- Polley, L. Navigating parasite webs and parasite flow: Emerging and re-emerging parasitic zoonoses of wildlife origin. Int. J. Parasitol. 2005, 35, 1279–1294. [Google Scholar] [CrossRef]
- Demirci, B.; Bedir, H.; Taskin Tasci, G.; Vatansever, Z. Potential mosquito vectors of Dirofilaria immitis and Dirofilaira repens (Spirurida: Onchocercidae) in Aras Valley, Turkey. J. Med. Entomol. 2021, 58, 906–912. [Google Scholar] [CrossRef]
- Tolnai, Z.; Széll, Z.; Sproch, Á.; Szeredi, L.; Sréter, T. Dirofilaria immitis: An emerging parasite in dogs, red foxes and golden jackals in Hungary. Vet. Parasitol. 2014, 203, 339–342. [Google Scholar] [CrossRef]
- Gomes-de-Sá, S.; Santos-Silva, S.; Moreira, A.S.; Barradas, P.F.; Amorim, I.; Cardoso, L.; Mesquita, J.R. Dirofilaria immitis antigenemia and microfilaremia in Iberian wolves and red foxes from Portugal. Parasit. Vectors 2022, 15, 119. [Google Scholar] [CrossRef]
- Sobotyk, C.; Nguyen, N.; Negrón, V.; Varner, A.; Saleh, M.N.; Hilton, C.; Tomeček, J.M.; Esteve-Gasent, M.D.; Verocai, G.G. Detection of Dirofilaria immitis via integrated serological and molecular analyses in coyotes from Texas, United States. Int. J. Parasitol. Parasites Wildl. 2022, 18, 20–24. [Google Scholar] [CrossRef]
- Tonev, A.S.; Kirkova, Z.; Iliev, P.T.; Roussenov, A.; Chaprazov, T.; Roydev, R.; Pirovski, N. Clinical case of life-threatening co-infection due to Dirofilaria immitis and Aelurostrongylus abstrusus in a cat: First report of feline heartworm disease in Bulgaria. Helminthologia 2021, 58, 106–114. [Google Scholar] [CrossRef]
- Molnár, V.; Pazár, P.; Rigó, D.; Máthé, D.; Fok, E.; Glávits, R.; Vajdovich, P.; Jacsó, O.; Balogh, L.; Sós, E. Autochthonous Dirofilaria immitis infection in a ferret with aberrant larval migration in Europe. J. Small Anim. Pract. 2010, 51, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Alho, A.M.; Marcelino, I.; Colella, V.; Flanagan, C.; Silva, N.; Correia, J.J.; Latrofa, M.S.; Otranto, D.; Madeira de Carvalho, L. Dirofilaria immitis in pinnipeds and a new host record. Parasit. Vectors 2017, 10, 142. [Google Scholar] [CrossRef] [PubMed]
- Selvachandran, A.; Foley, R.J. Subcutaneous and pulmonary dirofilariasis with evidence of splenic involvement. Case Rep. Pulmonol. 2016, 2016, 8212387. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Kim, C.H.; Yeom, B.W.; Park, S.H.; Choi, S.Y.; Choi, J.S. The first human case of hepatic dirofilariasis. J. Korean Med. Sci. 2002, 17, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Akao, N. Human dirofilariasis in Japan. Trop. Med. Health 2011, 39, 65–71. [Google Scholar]
- Požgain, Z.; Dulić, G.; Sego, K.; Blažeković, R. Live Dirofilaria immitis found during coronary artery bypass grafting procedure. Eur. J. Cardiothorac. Surg. 2014, 46, 134–136. [Google Scholar] [CrossRef] [PubMed]
- Theis, J.H.; Gilson, A.; Simon, G.E.; Bradshaw, B.; Clark, D. Case report: Unusual location of Dirofilaria immitis in a 28-year-old man necessitates orchiectomy. Am. J. Trop. Med. Hyg. 2001, 64, 317–322. [Google Scholar] [CrossRef]
- Malik, D.; Amaraneni, A.; Singh, S.; Roach, R. Man’s best friend: How humans can develop Dirofilaria immitis infections. IDCases 2016, 4, 43–45. [Google Scholar] [CrossRef]
- Merrill, J.R.; Otis, J.; Logan, W.D., Jr.; Davis, M.B. The dog heartworm (Dirofilaria immitis) in man. An epidemic pending or in progress? JAMA 1980, 243, 1066–1068. [Google Scholar] [CrossRef]
- Hamir, A.N. Heartworm (Dirofilaria immitis) in the brain of a dog. Vet. Rec. 1987, 120, 207–208. [Google Scholar] [CrossRef]
- Oh, H.W.; Jun, H.K.; You, M.J.; Hayasaki, M.; Song, K.H. Ectopic migration of an adult heartworm in a dog with dirofilariasis (case report). Korean J. Parasitol. 2008, 46, 171–173. [Google Scholar] [CrossRef] [PubMed]
- Grimes, J.A.; Scott, K.D.; Edwards, J.F. Aberrant heartworm migration to the abdominal aorta and systemic arteriolitis in a dog presenting with vomiting and hemorrhagic diarrhea. Can. Vet. J. 2016, 57, 76–79. [Google Scholar] [PubMed]
- Sevimli, F.K.; Kozan, E.; Bülbül, A.; Birdane, F.M.; Köse, M.; Sevimli, A. Dirofilaria immitis infection in dogs: Unusually located and unusual findings. Parasitol. Res. 2007, 101, 1487–1494. [Google Scholar] [CrossRef] [PubMed]
- Upchurch, D.A.; Ogden, D.M.; Baker, D.G. Bilateral femoral arterial dirofilariasis caused by Dirofilaria immitis in a dog. Vet. Rec. Case Rep. 2015, 3, e000184. [Google Scholar] [CrossRef]
- Liu, S.K.; Das, K.M.; Tashjian, R.J. Adult Dirofilaria immitis in the arterial system of a dog. J. Am. Vet. Med. Assoc. 1966, 148, 1501–1507. [Google Scholar]
- Bossie, A. Microfilaire de Dirofilaria immitis dans la moelle osseuse d’un chien. Arch. Roum. Pathol. Exp. Microbiol. 1968, 27, 651–654. [Google Scholar]
- Kaewthamasorn, M.; Assarasakorn, S.; Niwetpathomwat, A. Microfilaruria caused by canine dirofilariasis (Dirofilaria immitis): An unusual clinical presence. Comp. Clin. Pathol. 2008, 17, 61–65. [Google Scholar] [CrossRef]
- Monobe, M.M.; da Silva, R.C.; Araujo Junior, J.P.; Takahira, R.K. Microfilaruria by Dirofilaria immitis in a dog: A rare clinical pathological finding. J. Parasit. Dis. 2017, 41, 805–808. [Google Scholar] [CrossRef]
- Savic, S.; Vidic, B.; Grgic, Z.; Petrovic, T.; Potkonjak, A.; Cupina, A.; Vaselek, S.; Petric, D. Dirofilariosis and leishmaniasis in the Northern region of Serbia. In An Overview of Tropical Diseases; Amidou, S., Ed.; IntechOpen: Rijeka, Croatia, 2015; Chapter 6; pp. 107–125. [Google Scholar] [CrossRef]
- Ntais, P.; Christodoulou, V.; Dokianakis, E.; Antoniou, M. Leishmania infantum and Dirofilaria immitis coinfection in dogs in Greece. Parasitol. Open 2016, 2, e17. [Google Scholar] [CrossRef]
- Mendoza-Roldan, J.; Benelli, G.; Panarese, R.; Iatta, R.; Furlanello, T.; Beugnet, F.; Zatelli, A.; Otranto, D. Leishmania infantum and Dirofilaria immitis infections in Italy, 2009–2019: Changing distribution patterns. Parasit. Vectors 2020, 13, 193. [Google Scholar] [CrossRef]
- Oliveira, V.D.C.; Junior, A.A.V.M.; Ferreira, L.C.; Calvet, T.M.Q.; Dos Santos, S.A.; Figueiredo, F.B.; Campos, M.P.; Rodrigues, F.D.C.C.; de Oliveira, R.V.C.; de Lemos, E.R.S.; et al. Frequency of co-seropositivities for certain pathogens and their relationship with clinical and histopathological changes and parasite load in dogs infected with Leishmania infantum. PLoS ONE 2021, 16, e0247560. [Google Scholar] [CrossRef]
- Tasić-Otašević, S.; Savić, S.; Jurhar-Pavlova, M.; Stefanovska, J.; Stalević, M.; Ignjatović, A.; Ranđelović, M.; Gajić, B.; Cvetkovikj, A.; Gabrielli, S. Molecular survey of Dirofilaria and Leishmania species in dogs from Central Balkan. Animals 2022, 12, 911. [Google Scholar] [CrossRef]
- Alves Rodrigues, R.T.G.; Moreira Borges, O.M.; Pereira Dantas, A.K.F.; Tôrres, L.M.; Lucena, R.D.S.; Souza, A.P. Presença de Leishmania sp. e Dirofilaria immitis em tumor venéreo transmissível canino cutâneo. Acta Sci. Vet. 2019, 47, 399. [Google Scholar] [CrossRef]
- Harvey, J.W. Bone marrow examination. In Veterinary Hematology. A Diagnostic Guide and Color Atlas, 1st ed.; Harvey, J.W., Ed.; Elsevier Saunders: St. Louis, MO, USA, 2012; pp. 234–259. [Google Scholar] [CrossRef]
- Bauer, N.B.; Harr, K.E. Bone marrow evaluation. In Schalm’s Veterinary Hematology, 7th ed.; Brooks, M.B., Harr, K.E., Seelig, D.M., Wardrop, K.J., Weiss, D.J., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2022; pp. 1285–1294. [Google Scholar] [CrossRef]
- Solano-Gallego, L.; Miró, G.; Koutinas, A.; Cardoso, L.; Pennisi, M.G.; Ferrer, L.; Bourdeau, P.; Oliva, G.; Baneth, G. LeishVet guidelines for the practical management of canine leishmaniosis. Parasit. Vectors 2011, 4, 86. [Google Scholar] [CrossRef]
- Gradoni, L.; Pozio, E.; Bettini, S.; Gramiccia, M. Leishmaniasis in Tuscany (Italy). (III) The prevalence of canine leishmaniasis in two foci of Grosseto Province. Trans. R. Soc. Trop. Med. Hyg. 1980, 74, 421–422. [Google Scholar] [CrossRef]
- Piantedosi, D.; Veneziano, V.; Di Muccio, T.; Manzillo, V.F.; Fiorentino, E.; Scalone, A.; Neola, B.; Di Prisco, F.; D’Alessio, N.; Gradoni, L.; et al. Epidemiological survey on Leishmania infection in red foxes (Vulpes vulpes) and hunting dogs sharing the same rural area in Southern Italy. Acta Parasitol. 2016, 61, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.; Miletti, G.; Vangone, L.; Spadari, L.; Reccia, S.; Fusco, G. Heartworm disease (Dirofilaria immitis) in two roaming dogs from the urban area of Castel Volturno, Southern Italy. Front. Vet. Sci. 2019, 6, 270. [Google Scholar] [CrossRef] [PubMed]
- Macchioni, F.; Sed, G.; Cecchi, F. Canine filarial infections in an area of Central Italy (Tuscany-Latium border) historically free from the disease. Vet. Parasitol. Reg. Stud. Rep. 2020, 20, 100404. [Google Scholar] [CrossRef]
- Panarese, R.; Iatta, R.; Latrofa, M.S.; Zatelli, A.; Ćupina, A.I.; Montarsi, F.; Pombi, M.; Mendoza-Roldan, J.A.; Beugnet, F.; Otranto, D. Hyperendemic Dirofilaria immitis infection in a sheltered dog population: An expanding threat in the Mediterranean region. Int. J. Parasitol. 2020, 50, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Naucke, T.J.; Menn, B.; Massberg, D.; Lorentz, S. Sandflies and leishmaniasis in Germany. Parasitol. Res. 2008, 103, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Salomón, O.D.; Quintana, M.G.; Mastrángelo, A.V.; Fernández, M.S. Leishmaniasis and climate change-case study: Argentina. J. Trop. Med. 2012, 2012, 601242. [Google Scholar] [CrossRef]
- Tánczos, B.; Balogh, N.; Király, L.; Biksi, I.; Szeredi, L.; Gyurkovsky, M.; Scalone, A.; Fiorentino, E.; Gramiccia, M.; Farkas, R. First record of autochthonous canine leishmaniasis in Hungary. Vector Borne Zoonotic Dis. 2012, 12, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Kholoud, K.; Denis, S.; Lahouari, B.; El Hidan, M.A.; Souad, B. Management of leishmaniases in the era of climate change in Morocco. Int. J. Environ. Res. Public Health 2018, 15, 1542. [Google Scholar] [CrossRef]
- Morosetti, G.; Toson, M.; Trevisiol, K.; Idrizi, I.; Natale, A.; Lucchese, L.; Michelutti, A.; Ceschi, P.; Lorenzi, G.; Piffer, C.; et al. Canine leishmaniosis in the Italian northeastern Alps: A survey to assess serological prevalence in dogs and distribution of phlebotomine sand flies in the Autonomous Province of Bolzano-South Tyrol, Italy. Vet. Parasitol. Reg. Stud. Rep. 2020, 21, 100432. [Google Scholar] [CrossRef]
- Rochlin, I.; Ninivaggi, D.V.; Hutchinson, M.L.; Farajollahi, A. Climate change and range expansion of the Asian tiger mosquito (Aedes albopictus) in Northeastern USA: Implications for public health practitioners. PLoS ONE 2013, 8, e60874. [Google Scholar] [CrossRef] [PubMed]
- Petruff, T.A.; McMillan, J.R.; Shepard, J.J.; Andreadis, T.G.; Armstrong, P.M. Increased mosquito abundance and species richness in Connecticut, United States 2001–2019. Sci. Rep. 2020, 10, 19287. [Google Scholar] [CrossRef] [PubMed]
- Gorris, M.E.; Bartlow, A.W.; Temple, S.D.; Romero-Alvarez, D.; Shutt, D.P.; Fair, J.M.; Kaufeld, K.A.; Del Valle, S.Y.; Manore, C.A. Updated distribution maps of predominant Culex mosquitoes across the Americas. Parasit. Vectors 2021, 14, 547. [Google Scholar] [CrossRef]
- Morchón, R.; Carretón, E.; González-Miguel, J.; Mellado-Hernández, I. Heartworm disease (Dirofilaria immitis) and their vectors in Europe—New Distribution Trends. Front. Physiol. 2012, 3, 196. [Google Scholar] [CrossRef]
- Montoya-Alonso, J.A.; Carretón, E.; Morchón, R.; Silveira-Viera, L.; Falcón, Y.; Simón, F. The impact of the climate on the epidemiology of Dirofilaria immitis in the pet population of the Canary Islands. Vet. Parasitol. 2016, 216, 66–71. [Google Scholar] [CrossRef]
- Drake, J.; Wiseman, S. Increasing incidence of Dirofilaria immitis in dogs in USA with focus on the southeast region 2013–2016. Parasit. Vectors 2018, 11, 39. [Google Scholar] [CrossRef]
- Polizopoulou, Z.S.; Koutinas, A.F.; Saridomichelakis, M.N.; Patsikas, M.N.; Leontidis, L.S.; Roubies, N.A.; Desiris, A.K. Clinical and laboratory observations in 91 dogs infected with Dirofilaria immitis in northern Greece. Vet. Rec. 2000, 146, 466–469. [Google Scholar] [CrossRef]
- Vieira, A.L.; Vieira, M.J.; Oliveira, J.M.; Simões, A.R.; Diez-Baños, P.; Gestal, J. Prevalence of canine heartworm (Dirofilaria immitis) disease in dogs of central Portugal. Parasite 2014, 21, 5. [Google Scholar] [CrossRef] [PubMed]
- Falcón-Cordón, Y.; Tvarijonaviciute, A.; Montoya-Alonso, J.A.; Muñoz-Prieto, A.; Caro-Vadillo, A.; Carretón, E. Evaluation of acute phase proteins, adiponectin and endothelin-1 to determine vascular damage in dogs with heartworm disease (Dirofilaria immitis), before and after adulticide treatment. Vet. Parasitol. 2022, 309, 109759. [Google Scholar] [CrossRef]
- Car, S.; Croton, C.; Haworth, M. Pseudohypoadrenocorticism in a Siberian Husky with Trichuris vulpis infection. Case Rep. Vet. Med. 2019, 2019, 3759683. [Google Scholar] [CrossRef]
- Saridomichelakis, M.N.; Xenoulis, P.G.; Chatzis, M.K.; Kasabalis, D.; Steiner, J.M.; Suchodolski, J.S.; Petanides, T. Thyroid function in 36 dogs with leishmaniosis due to Leishmania infantum before and during treatment with allopurinol with or without meglumine antimonate. Vet. Parasitol. 2013, 197, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Tabar, M.D.; Altlet, L.; Martínez, V.; Roura, X. Wolbachia, filariae, and Leishmania coinfection in dogs from a Mediterranean area. J. Small Anim. Pract. 2013, 54, 174–178. [Google Scholar] [CrossRef]
- Morales-Yuste, M.; Martín-Sánchez, J.; Corpas-Lopez, V. Canine leishmaniasis: Update on epidemiology, diagnosis, treatment, and prevention. Vet. Sci. 2022, 9, 387. [Google Scholar] [CrossRef]
- Paparcone, R.; Fiorentino, E.; Cappiello, S.; Gizzarelli, M.; Gradoni, L.; Oliva, G.; Foglia Manzillo, V. Sternal aspiration of bone marrow in dogs: A practical approach for canine leishmaniasis diagnosis and monitoring. J. Vet. Med. 2013, 2013, 217314. [Google Scholar] [CrossRef] [PubMed]
- Marks, S.L.; Kendall, A. Anemia Caused by Primary Bone Marrow Diseases in Animals. Available online: https://www.msdvetmanual.com/circulatory-system/anemia/anemia-caused-by-primary-bone-marrow-diseases-in-animals (accessed on 6 March 2023).
- Magnis, J.; Lorentz, S.; Guardone, L.; Grimm, F.; Magi, M.; Naucke, T.J.; Deplazes, P. Morphometric analyses of canine blood microfilariae isolated by the Knott’s test enables Dirofilaria immitis and D. repens species-specific and Acanthocheilonema (syn. Dipetalonema) genus-specific diagnosis. Parasit. Vectors 2013, 6, 48. [Google Scholar] [CrossRef]
- Borthakur, S.K.; Deka, D.K.; Islam, S.; Sarmah, P.C. Occult dirofilariosis in dogs of North Eastern region in India. J. Arthropod Borne Dis. 2015, 10, 92–97. [Google Scholar]
- Henry, L.G.; Brunson, K.J.; Walden, H.S.; Wenzlow, N.; Beachboard, S.E.; Barr, K.L.; Long, M.T. Comparison of six commercial antigen kits for detection of Dirofilaria immitis infections in canines with necropsy-confirmed heartworm status. Vet. Parasitol. 2018, 254, 178–182. [Google Scholar] [CrossRef]
- Albonico, F.; Loiacono, M.; Gioia, G.; Genchi, C.; Genchi, M.; Mortarino, M. Rapid differentiation of Dirofilaria immitis and Dirofilaria repens in canine peripheral blood by real-time PCR coupled to high resolution melting analysis. Vet. Parasitol. 2014, 200, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Cornegliani, L.; Di Cesare, A.; Aste, G.; Traversa, D.; Di Giulio, E.; Santori, D.; Vercelli, A.; Pampurini, F.; Boari, A. Simultaneous infection by Dirofilaria repens and Leishmania infantum in a dog. Rev. Veterinaire Clin. 2015, 50, 59–63. [Google Scholar] [CrossRef]
- Pradhan, S.; Lahiri, V.L.; Elhence, B.R.; Singh, K.N. The microfilariae of Wuchereria bancrofti in bone marrow smears. Am. J. Trop. Med. Hyg. 1976, 25, 199–200. [Google Scholar] [CrossRef] [PubMed]
- Tummidi, S.; Patro, M.K.; Bal, A.K.; Choudhury, A. Microfilariae in a bone marrow aspirate. BMC Res. Notes 2016, 9, 256. [Google Scholar] [CrossRef] [PubMed]
- Rahman, K.; Harankhedkar, S.; Gupta, R.; Gupta, T.; Sharma, S.; Nityanand, S. Microfilariae in bone marrow aspirate of a case of myelofibrosis: A cause or coincidence? J. Cancer Res. Ther. 2020, 16, 164–166. [Google Scholar] [CrossRef]
- Ahluwalia, C.; Choudhury, M.; Bajaj, P. Incidental detection of microfilariae in aspirates from Ewing’s sarcoma of bone. Diagn. Cytopathol. 2003, 29, 31–32. [Google Scholar] [CrossRef]
- Andola, S.K.; Naik, A.A. Microfilaria and filarial granulomas from fine needle aspirates: A study of 25 cases. Southeast Asian J. Trop. Med. Public Health 2011, 42, 1351–1358. [Google Scholar]
- Chakraborty, S.; Saha, M.; Pradhan, S.G.; Biswas, S. Microfilaria infection in metastatic node in a case of breast carcinoma. J. Midlife Health 2019, 10, 153–155. [Google Scholar] [CrossRef]
- Dhanya, C.S.; Jayaprakash, H.T. Microfilariae, a common parasite in an unusual site: A case report with literature review. J. Clin. Diagn. Res. 2016, 10, ED08–ED09. [Google Scholar] [CrossRef]
- Pal, S.; Mondal, S.; Pradhan, R.; Bose, K.; Chakrabarti, S.; Sikder, M. Cytological findings of microfilariae in different sites: A retrospective review of 22 cases from endemic region. Trop. Parasitol. 2018, 8, 24–28. [Google Scholar] [CrossRef]
- Vasantham, V.; Yadav, S.K.; Sarin, N.; Singh, S.; Pruthi, S.K. Incidental detection of microfilaria in cyst fluid of mucinous cystadenocarcinoma of ovary: A rare case report. Int. J. Surg. Case Rep. 2020, 70, 56–59. [Google Scholar] [CrossRef]
- American Heartworm Society. Current Canine Guidelines for the Prevention, Diagnosis, and Management of Heartworm (Dirofilaria immitis) Infection in Dogs. Available online: https://d3ft8sckhnqim2.cloudfront.net/images/pdf/2020_AHS_Canine_Guidelines.pdf?1580934824 (accessed on 7 March 2023).
- Velez, R.; Gállego, M. Commercially approved vaccines for canine leishmaniosis: A review of available data on their safety and efficacy. Trop. Med. Int. Health 2020, 25, 540–557. [Google Scholar] [CrossRef]
- Chalifoux, N.V.; Parker, S.E.; Cosford, K.L. Prognostic indicators at presentation for canine parvoviral enteritis: 322 cases (2001–2018). J. Vet. Emerg. Crit. Care (San Antonio) 2021, 31, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Machado, I.C.; Nunes, T.; Maximino, M.; Malato, J.; Tavares, L.; Almeida, V.; Sepúlveda, N.; Gil, S. Epidemiologic factors supporting triage of infected dog patients admitted to a veterinary hospital biological isolation and containment unit. Vet. Sci. 2023, 10, 186. [Google Scholar] [CrossRef] [PubMed]
- Orusa, T.; Orusa, R.; Viani, A.; Carella, E.; Enrico, B.M. Geomatics and EO data to support wildlife diseases assessment at landscape level: A pilot experience to map infectious keratoconjunctivitis in chamois and phenological trends in Aosta Valley (NW Italy). Remote Sens. 2020, 12, 3542. [Google Scholar] [CrossRef]
- Pacheco, G. Relationship between the number of circulating microfilariae and the total population of microfilariae in a host. J. Parasitol. 1974, 60, 814–818. [Google Scholar] [CrossRef] [PubMed]
- Ludders, J.W.; Grauer, G.F.; Dubielzig, R.R.; Ribble, G.A.; Wilson, J.W. Renal microcirculatory and correlated histologic changes associated with dirofilariasis in dogs. Am. J. Vet. Res. 1988, 49, 826–830. [Google Scholar]
- Sacks, B.N.; Blejwas, K.M. Effects of canine heartworm (Dirofilaria immitis) on body condition and activity of free-ranging coyotes (Canis latrans). Can. J. Zool. 2000, 78, 1042–1051. [Google Scholar] [CrossRef]
- Sharma, S.; Rawat, A.; Chowhan, A. Microfilariae in bone marrow aspiration smears, correlation with marrow hypoplasia: A report of six cases. Indian J. Pathol. Microbiol. 2006, 49, 566–568. [Google Scholar]



| Parameters and Units | Reference Interval | Dh * | Drv ** |
|---|---|---|---|
| RBC M/mL | 5.65–8.87 | 3.84 | 3.64 |
| HCT % | 37.3–61.7 | 23.5 | 23.2 |
| HGB g/dL | 13.1–20.5 | 8.7 | 8.6 |
| MCV fL | 61.6–73.5 | 61.6 | 63.7 |
| MCH pg | 21.2–25.9 | 22.7 | 23.6 |
| MCHC g/dL | 32.0–37.9 | 37.0 | 37.1 |
| RDW % | 13.6–21.7 | 17.3 | 20.0 |
| Retics K/mL | 10.0–110.0 | 49.9 | 32.0 ^ |
| Retic-HGB pg | 22.3–29.6 | 28.8 | 17.5 |
| Notes on RBCs | None | Howell–Jolly bodies ++ | |
| WBC K/mL | 5.05–16.76 | 28.58 | 14.8 |
| NEU seg K/mL | 3.7–11.9 | 24.73 | 12.28 |
| NEU band K/mL | 0.0–0.3 | Flag | 1.33 |
| EOS K/mL | 0.1–1.35 | 0.01 | 0.15 |
| BAS K/mL | 0.0–0.1 | 0.05 | 0.0 |
| LYM K/mL | 0.7–5.1 | 1.44 | 0.15 |
| MON K/mL | 0.2–1.5 | 2.35 | 0.89 |
| Notes on WBCs | None | None | Toxic neutrophils +/−, activated monocytes ++ |
| PLT K/mL | 148–484 | 72 | 79 |
| MPV fL | 8.7–13.2 | 12.6 | 15.1 |
| PDW fL | 9.1–19.4 | ND | ND |
| PCT % | 0.14–0.46 | 0.09 | 0.12 |
| PLT estimate | Adequate | ND | Inadequate |
| Other notes | None | One microfilaria in each of the two smears examined |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lensi, I.; Lubas, G.; Papini, R.A. Incidental Finding of Dirofilaria immitis (Spirurida: Onchocercidae) Microfilariae in the Bone Marrow of a Dog with Mixed Leishmania infantum-Dirofilaria immitis Infection. Zoonotic Dis. 2023, 3, 162-175. https://doi.org/10.3390/zoonoticdis3020013
Lensi I, Lubas G, Papini RA. Incidental Finding of Dirofilaria immitis (Spirurida: Onchocercidae) Microfilariae in the Bone Marrow of a Dog with Mixed Leishmania infantum-Dirofilaria immitis Infection. Zoonotic Diseases. 2023; 3(2):162-175. https://doi.org/10.3390/zoonoticdis3020013
Chicago/Turabian StyleLensi, Ilaria, George Lubas, and Roberto Amerigo Papini. 2023. "Incidental Finding of Dirofilaria immitis (Spirurida: Onchocercidae) Microfilariae in the Bone Marrow of a Dog with Mixed Leishmania infantum-Dirofilaria immitis Infection" Zoonotic Diseases 3, no. 2: 162-175. https://doi.org/10.3390/zoonoticdis3020013