1. Introduction
Human brain organoids are artificially grown, small-scale tissue constructs that resemble the immature brain. They are produced from human pluripotent stem cells (embryonic stem cells (ESCs) derived from the inner cell mass of a pre-implant blastocyst or induced pluripotent stem cells ((iPSCs);
Figure 1) derived from adult somatic cells) that self-pattern into three-dimensional (3D) multicellular neural tissues with defined compartments; including radially arranged neural tube-like neuroepithelial structures and immature cortical plate. As such, human brain organoids offer significant potential for studying aspects of human brain biology by recapitulating some of the features of development and maturation (particularly corticogenesis), including normal function and dysfunction.
While brain organoids are currently limited to being only a few millimetres in size, they display remarkable anatomical and functional complexity, offering insights into brain and disease and related therapeutics’ modelling such as pharmaceuticals’ responsivity. Research also has the potential to extend to resolving the evolutionary differences between the human brain and other species, in terms of gross morphology, size and underlying cellular and molecular complexity [
1], and has involved experimentation by fusing different neural organoids in vitro and in vivo chimeric research [
2,
3].
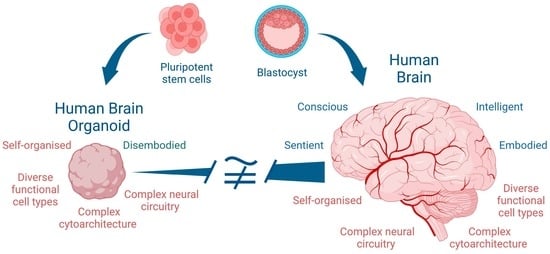
As human brain organoids are composed of masses of neural cells that may be organised in a manner resembling brain tissue, they offer researchers the opportunity to study the development and interactions of cells which may provide better proxies to the human brain and diseases and disorders than animal models, whilst also potentially decreasing the need for animal models in research. Human brain organoids are disembodied (living, but ex vivo) engineered brain tissue, they can be generated from iPSCs and they are not developing embryos or foetuses. Therefore, their use is thought to avoid many ethical concerns and regulatory limitations associated with human embryo research and research on human brains in live humans. As the generation of human brain organoids involves the use of stem cells and techniques adopted from other disciplines, such as tissue engineering, many of the ethical and regulatory concerns raised by human brain organoids are the same as those raised in other forms of biomedical research and translation. These include procurement of cells and consent to the use of cell lines derived from tissue donors and research oversight [
2,
4,
5,
6,
7].
An ethical concern
peculiar to human brain organoids is whether they are or could become capable of supporting consciousness and so merit some special moral consideration. Researchers have raised concerns that with further technical development, human brain organoids could attain sentience, in the sense of being able to perceive the environment and to experience subjective phenomenal states or sensations, such as pain and suffering or pleasure and comfort [
2,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17]. The idea that human brain organoids could become sentient arises from the structural similarity of some human brain organoids to human brains, their ability to self-organise, the rapid development of techniques for developing organoids and recent cellular correlates of intelligence and sentience for in vitro neuronal cell cultures [
18]. In addition to sentience, concerns have been raised about whether human brain organoids could develop higher cognitive abilities, including memory formation and retrieval, thoughts and self-awareness [
7,
8,
19].
If human brain organoids are or may become conscious, consideration of their moral status and the forming of appropriate regulations to protect their welfare follows [
2,
7,
8,
9,
10,
11,
12,
13,
14,
15,
17,
20]. Evidence of organoid consciousness could generate obligations to respect their welfare and to reconceptualise related moral or legal obligations to ensure a duty of care, including practices around stewardship, ownership, death, consent, post research handling and data and animal–human research [
8,
19,
20]. Whilst the majority of researchers propose that in their current state of development the likelihood that human brain organoids possess consciousness is extremely low [
2,
7,
9,
21], there is, however, a growing sense of urgency among researchers that as the science develops, the issue of human-brain organoid consciousness needs to be addressed. The concern is summed up well by Farahany et al.: “the closer the proxy gets to a functioning human brain, the more ethically problematic it becomes” (p. 429) [
8].
Despite these concerns, identifying the presence of consciousness in human brain organoids is complicated by several factors. Consciousness is a highly contested topic among neuroscientists, cognitive scientists and philosophers and there is no agreed definition of consciousness. As a result, it is not clear that researchers are talking about the same thing and that human brain organoids could be considered conscious on some definitions but not on others. These conceptual disagreements generate a potential lack of trust in the research agenda [
2,
22]. Secondly, given human brain organoids are disembodied, there is no practical way to identify evidence of consciousness in a human brain organoid as we might in humans or other animals, for example, through interrogation and/or the assessment of behavioural indicators, such as responses to pain or injury. What would count as evidence of organoid consciousness is an emerging area of research.
In the face of this uncertainty, Greely argues researchers face an “onrushing ethical dilemma” [
20]—we “can’t rule out the possibility that sufficiently sophisticated organoids are, or will soon be, sentient; capable of having feelings with positive or negative quality, such as feelings of pain and pleasure. If they are sentient then there are moral limits on their research use, and regulation is urgently needed to prevent research overstepping those limits” (p. 56) [
16].
This paper clarifies the morally relevant aspects of human consciousness, phenomenal experience and embodied development and explores the empirical basis of consciousness. These then provide the basis for developing a defensible framework for informed decision-making on the moral significance and utility of brain organoids, which can guide the regulation and future research of these novel biological systems. In the first section, we review the science of human brain organoids, indicating the present state of research, as well as emerging developments in this area of science. In the second section, we unpack claims raised about human-brain organoid consciousness through philosophical discussion of the moral significance of consciousness to inform concerns about human-brain organoid consciousness and moral status. In the third section, we critically review emerging neuroscientific approaches to defining and measuring consciousness in human brain organoids. We ask what would count as evidence of consciousness in human brain organoids and expose an explanatory gap between observation and attribution of consciousness. In the concluding section, we bring these (philosophical and scientific) discussions together to assess the challenges related to the regulation of human brain organoids based on consciousness. We show that, whilst we lack evidence to support a claim of consciousness in human brain organoids that matters morally, gaps in certainty for establishing consciousness in human brain organoids present challenges for developing regulations.
Figure 1.
Human iPSC-derived human brain organoids, (
A) comprising complex cellular and regional heterogeneity, (
B) generated in large numbers using 3D-printed hydrogel arrays, and (
C) placed on a microelectrode array (MEA) for extracellular electrophysiological recordings [
23].
Figure 1.
Human iPSC-derived human brain organoids, (
A) comprising complex cellular and regional heterogeneity, (
B) generated in large numbers using 3D-printed hydrogel arrays, and (
C) placed on a microelectrode array (MEA) for extracellular electrophysiological recordings [
23].
2. Review of the Science of Human Brain Organoids: The Present State of Research and Emerging Developments
Human brain organoids provide a remarkable opportunity to model prenatal human brain biology in vitro by recapitulating features of in utero molecular, cellular and systems biology (
Figure 2). Since the reports by Preynat-Seauve and colleagues [
24] and Lancaster and colleagues [
25,
26], there have been an increasing number of research papers describing the generation of brain organoids, also termed cerebral organoids or spheroids. The reports variously detail the cell composition and function, structural organization and in some cases perturbation of biological processes resembling in vivo diseased brain tissue [
27,
28,
29,
30,
31,
32,
33,
34,
35,
36,
37]. Collectively, the studies provide compelling evidence for the utility of the platform, especially for the study of human corticogenesis, posited to be analogous to a 9–10-week-old embryonic brain, and a biologically relevant alternative to conventional animal models and human brain tissues obtained post-mortem or through live tissue-ectomy.
In recognising the potential of organoid modelling, it is important to understand that current systems do not replicate the patterning and environmental cues found in an intact embryo. Indeed, all studies using brain organoids have been limited to researching early developmental events in the brain, being less relevant to later foetal and adult brain development. A principal impediment to growing later-stage organoids is the absence of critical homeostatic components such as vascularization for optimal and scalable gas exchange, nutrient supply and waste removal, as well as a sensory–neural system for sensory–neural input. For the latter, external stimuli typically influence neuroplasticity and regional specificity by appropriately affecting gene expression and cell phenotypes to promote neural pathways underlying pre- and post-natal brain maturation and function.
Notwithstanding the challenges to improving current systems, efforts are being made to evolve organoids towards more complete and mature brain-like tissues through extended culture (e.g., more than 9 months) [
36], use of biomaterials [
23,
31,
33], organoid fusion [
27] and networking [
23] and vascularization [
38,
39]. Moreover, fusion and/or networking of organoids are strategies for increasing the level of complexity and better recapitulating in vivo human brain form and function, in addition to enabling novel molecular and cellular studies of organ and tissue development, extending to genetic manipulation [
27]. Such advances (inclusive of simplifying protocols) serve to expand the use of the technology in both basic and applied research and medicine, with recent increased uptake after a period of relative inactivity following initial reports.
The value of brain organoids as 3D models of brain development and function is underscored by being derived from either healthy or diseased human donor cells, with the opportunity to recapitulate previously intractable human neurobiology in vitro, investigate pathologies and test prospective treatments, as well as in vivo assessment via animal implantation. Examples include modelling human neuromelanin production, function and potential neurotoxicity, with understanding till now constrained by limited post-mortem human midbrain tissue studies [
29], and disease modelling with RNA interference and patient-specific iPSCs for microcephaly [
26]. Microcephaly is a rare neurological disorder characterised by abnormal brain development culminating in smaller brain size. Both genetic and environmental factors can cause the condition, with the latter modelled by Zika virus (ZIKV) infection of forebrain organoids resulting in reduced organoid size and neuronal cell-layer volume, concurrent cell death and decreased cell proliferation, as well as dilated lumen of ventricular structures [
35]. Utility of organoids for testing drugs to treat ZIKV infection has also been described, with compounds mitigating ZIKV-induced cytopathy, extending to Toll like-Receptor 3 (TLR3)-mediated apoptosis and tissue reduction [
40,
41]. Such findings call attention to the need for studying human biological systems to understand human development and disease, against which putative therapeutics should be evaluated. Whether or not this precludes grafting human organoids to animal hosts for modelling under in vivo physiological conditions remains to be determined, in spite of recent notable findings of functional tissue integration within a mouse brain, extending to vascularisation and extensive axonal outgrowth with synaptic assemblies between implanted organoids and host tissue [
38].
There are indubitably advantages to employing the brain organoid platform for research and development, offering an unprecedented opportunity to examine aspects of human organogenesis, in situ tissue cellular heterogeneity, organisation and function, and tissue dynamics and homeostasis. Notwithstanding, the field is in its infancy with the technology still in a developmental phase, requiring improved reliability through the standardisation of protocols that ideally permit structural and functional maturation of organoids, including gross anatomical- and cyto-architecture, to accurately recapitulate features of human brain development. Importantly, the refinement of organoid protocols is dependent upon reliable and advanced tools to characterise and understand the complex macro- and micro-biological features of 3D living tissue structures. There are many conventional assays that can be applied to be informative, such as classical tissue sectioning with immunohistochemistry and wide-field fluorescence microscopy, or molecular assays for gene and protein expression. However, it is essential to use methods more pertinent to the complexity of 3D tissue anisotropy. For example, 4D live-imaging of dynamic cellular and subcellular processes using confocal live-cell microscopy allows for spatio-temporal characterisation of organoid molecular and cellular biology while recording multichannel electrophysiological data to represent 3D tissue anisotropy more naturally and recapitulates the neuronal function and network formation of natural brain tissue [
42,
43]. Critically, there is a need for systematic in-depth probing of organoid induction and maturation, enabling a better understanding of the limit(s), immediate application(s) and options for improvement of protocol(s) towards consistent and accurate modelling.
3. Consciousness, Moral Status and Human Brain Organoids: Unpacking Claims about Consciousness and Moral Concern
Given that human brain organoids replicate, in some measure, human embryonic brain development and display patterns of self-generated electrical activity and a capacity for self-organisation, it seems to some commentators natural to be concerned with assessing whether human brain organoids are or may become capable of supporting consciousness [
2,
7,
8,
9,
10,
11,
12,
13,
14,
15,
17,
20]. The concern is raised that human brain organoids might attain sentience—that is, the ability of a living being to perceive its environment and to experience subjective phenomenal states or sensations, such as pain and suffering or pleasure and comfort. Further, some researchers have raised the concern that human brain organoids could develop more sophisticated capacities, such as “higher levels of cognition, including memory formation and retrieval, thoughts and some perception of agency and self-awareness” (p. 430) [
8]. These are capacities associated with higher level mammals and human beings, although recent research by Kagan and colleagues suggests cellular correlates of intelligence and sentience for an in vitro “DishBrain” neuronal cell culture system [
18]. The circumstance of a self-aware brain unable to communicate this situation has been described by some researchers as akin to a “living hell” [
7,
9,
10,
14,
20]. If human brain organoids have the capacity to support consciousness and capacities associated with sentience, this would raise questions about the moral status of human brain organoids, that their welfare should be considered, including the introduction of limits to their use in research.
Why is consciousness thought to give rise to moral concern and how would this attribution apply in the case of human brain organoids? Moral status is an important concept in philosophy for determining how an entity should be treated. “[A]n entity has moral status if and only if it or its interests morally matter to some degree for the entity’s own sake” [
44]. On some approaches, moral status is an “all or nothing” property—an entity has moral status, or it does not. On approaches popular in bioethics and research ethics, moral status is a property thought to come in degrees. Such approaches distinguish entities that possess Full Moral Status—moral agents that have a right to non-interference and can function as the bearers of moral obligations towards others—from moral patients—entities towards which moral agents can have moral responsibilities that need not themselves be capable of moral agency and lack autonomy and the ability to take moral responsibility. Although subject to critique, typically, human beings and some non-human animals are considered to possess full moral status because of sophisticated cognitive capacities or the capacity to develop these capacities, or because of membership of a cognitively sophisticated species.
Moral status and the different kinds of moral consideration afforded to an entity are differentiated in terms of the kind of consciousness possessed by an entity. In discussions of moral status, philosophers distinguish between different aspects of consciousness, including self-consciousness, phenomenal consciousness and access consciousness. Self-consciousness involves the possession of a concept of self and personal identity and involves sophisticated cognitive functioning. Phenomenal consciousness is subjective experience with qualitative content, for example, pain or pleasure. The subjective awareness of experiences by conscious individuals are known as qualia. Phenomenal consciousness, in philosophical discussion, is the “what it is like to be that something”—there is a way to be a phenomenally conscious entity. Given the subjective nature of phenomenal states, facts about an experiencer’s conscious states are inherently subjective (in the sense of being private to the individual having the conscious experience) and cannot be fully described or grasped by others, including other species [
45,
46]. We can describe or observe the sound, smell or taste of something, but not the subjective experience of another experiencer having that experience, of hearing that sound, smelling or tasting that thing. Phenomenal consciousness is often contrasted with access consciousness, the ability to use reasoning under direct conscious control by action or speech and which involves global availability of information to cognitive systems [
47,
48]. Access consciousness, in addition to phenomenal consciousness, is required for personhood and full moral status [
48].
The question of what properties—sentience, agency, rationality—are necessary for viewing an entity as meriting moral concern is contested within moral philosophy. Philosophers argue about which particular properties of humans are key to having moral status or being owed special treatment by virtue of being an entity with full moral status equivalent to that afforded to an adult human. Nonetheless, many would agree that subjective or phenomenal experience is minimally required for a being to merit moral consideration and that recognition of a being’s self-awareness is often thought to motivate further moral responses from others. In research ethics, whilst also contested, adult human beings and high order non-human animals are considered moral agents and as having complex interests that endure over time, while other animals are generally thought of as being moral patients with more limited interests (e.g., to avoid suffering). Contemporary philosophical accounts of consciousness, drawing on work in developmental neuroscience and phenomenology, emphasise the role of embodiment and embeddedness in a linguistic culture to both the development of, and experience of, consciousness and argue for the necessity of embodiment for the development of sentience, agency and self-awareness.
How do these discussions about consciousness and moral status inform concerns raised by researchers about human brain organoids? The onset of phenomenal consciousness (sentience) in human brain organoids is identified as a “clear” moral threshold or the point at which a human brain organoid should be afforded a degree of moral status and is due moral consideration [
7,
9,
10,
11,
12,
13,
20,
49]. Contrasting approaches note that consciousness is not the sole or most appropriate indicator of moral status and that there are other indicators of moral status. Such approaches extend moral status to entities that have a human origin and that have the potential to generate human beings [
12,
47]. On this basis, Hostiuc et al. assert that the reliance on sentience and the ability to experience pain may miss pertinent concerns with respect to the moral status of human brain organoids [
49]. If there is “something it is like to be” a human brain organoid, then their welfare would need to be considered and their use in research would need to occur within ethical boundaries, just as research with non-human animals and human foetuses is subject to ethical restrictions. Should human brain organoids be entitled to special treatments and rights not afforded to other clumps of living cells?
Anticipating capacities for sentience in, and consequent moral consideration towards, human brain organoids is not straightforward. This is despite recent claims of cellular correlates of senescence for neuronal cell cultures [
18]. Unlike in vivo human consciousness, human brain organoids are grown in vitro and are kept alive in a bioreactor [
14]. It is difficult to make sense of the idea of bodiless self-aware brains. In their current development, they lack sensory input and output mechanisms as well as a precortex; lacking these structures, it is hard to imagine how bunches of brain cells could think and perceive. The attribution of these capacities to human brain organoids presupposes aspects of in vivo development and obscure significant differences between human brain organoids and people [
14,
50]. Human brain organoids do not develop in the same way as human foetuses, and they lack a body and uterine conditions. They lack an embodied and socially interactive engagement with the world. The fact that human brain organoids are derived from human cells and appear to function in some ways like a foetal brain is not sufficient to establish that they should be viewed as having the same moral status as other humans, even if they could have subjective experience. Although the synthetic creation of human brain organoids shares some of the ethical features familiar to human cloning and embryo research, their derivation ex vivo from stem cell lines demonstrates that the process of development does not parallel the early development of the brain in the human embryo and that the self-organisation of brain organoids occurs outside of any intact embryo. If it were possible to maintain a human brain organoid until the brain was fully developed, it would never, itself, become a living human being’s brain without (somehow) being implanted in a living human body.
That human brain organoids are derived from human cells and comprise neural cells, associated with the developed human brain, personal identity and the moral status of living humans, “may be unduly prejudicing peoples’ concerns about the moral status of these in vitro models” (p. 10) [
2]. Concerns about consciousness in human brain organoids may be inflated by a commitment to the specialness of human consciousness with cultural tropes driving the intuition that human brain organoids matter morally [
2,
50]. Being composed of human neural cells does not give neural tissue moral status, nor would it be sufficient to justify preventing the use of human brain organoids in research.
Identifying the differences between in vitro and in vivo brains provides good reasons to resist over-emphasising this ethical concern about human-brain organoid consciousness at this time. In the absence of these features and of embodied development, there is no reason to believe that sentience would either be present or develop in a human brain organoid. Human brain organoids are comprised of human neural cells but are not embodied, claims about the “possible emergence of consciousness” are overstated and the attribution of moral status, including of personhood, is unwarranted.
4. Consciousness, Evidence and Human Brain Organoids: A Critical Review of Approaches to Evidence of Consciousness
The ethical question of whether human brain organoids could be conscious is linked to the epistemological question of how would we know [
51]? Neuroscientists propose theories of consciousness and look to the brain and brain processes for evidence of consciousness. Consciousness is a highly contested topic among neuroscientists, cognitive scientists and philosophers and there is no agreed definition of consciousness. In addition, as discussed in the previous section, phenomenal consciousness is a subjective state. Investigators cannot directly experience another entity’s subjective states. Philosophers refer to the challenge of determining whether the observable behaviour of another person means that they are having a particular mental experience as the “problem of other minds.” Conscious experience is largely private and the phenomenal properties of our mental states are not observable (e.g., the experience of the taste of chocolate that a particular person is experiencing at a given time). Consciousness in this context is something like the possession of an inner life, the way things are experienced by that being, and so inherently private, even if one uses behavioural cues to infer their existence. Neuroscientists rely on the subject’s observable behaviour to track consciousness empirically. Identifying the presence of consciousness in human brain organoids is complicated because they have ability to exhibit kinds of behaviours that suggest conscious experience used in non-human animals such as avoidance of pain stimuli and blinking. To find evidence of consciousness in human brain organoids may rely on other cues. In this section, we critically review approaches to developing empirical indicators of consciousness in human brain organoids, including inferences from brain activity and development and approaches to develop a metric based on non-responsive patients.
Conscious experience is explained in terms of brain activity. Consciousness is thought to be associated with high-frequency (gamma band) oscillations in brain activity This theory arises from proposals that gamma oscillations may link information represented in different parts of the brain into a unified experience [
52]. Researchers have reported gamma oscillations in 6–10 months-old organoids and reported similarities between chaotic bursts of synchronized electrical activity in the EEG (brain wave) patterns of brain organoids and late preterm-infants born at 25–39 weeks post-conception [
53]. The findings could be indicative of progress towards greater complexity within the organoid and have raised discussions about the onset of foetal pain.
Caution should be exercised with respect to interpreting these findings. There is consensus that neonates feel pain, however there is lack of agreement on when foetal pain arises [
54]. Whilst there is some consensus that foetal pain does not arise until the third trimester or post birth, some researchers argue for the development of foetal pain and consciousness as early as the second trimester, at about 20 weeks. Johnson et al. distinguishes neociception—”the object of sensory physiology” or “neural processes of encoding noxious stimuli”—from the subjective experience of pain—a sensation that is subjectively and phenomenally perceived to be painful, which requires more complex higher-order cognitive functions [
50]. Accounts that argue for the early presence of foetal pain take the presence/development of neociceptor and neocicpetive pathways in the developing foetus as evidence of the capacity for foetal pain. More significantly, that the brain waves of the organoids in this research appear similar to those in premature babies does not mean the organoids have similar experiences [
2,
55]. If human brain organoids could be developed to an equivalent level of brain development as human foetal brains, where foetuses start to show pain responses, then we should not treat human brain organoids in ways that cause them to experience pain, but brain organoids are unlike human foetuses in that they are ex vivo; they lack bodies. Furthermore, the brain itself is without pain receptors and as such does not feel pain, though future research may bypass this constraint, for example research which fuses different kinds of organoids. That these patterns are present in human brain organoids does not provide evidence of consciousness.
Some researchers suggest that brain region specificity will be a good indicator of consciousness in human brain organoids, with the development of higher brain areas considered more promising indicators, especially the prefrontal cortex, which is involved in a range of executive (higher-order) functions. There is substantial evidence that a “top-down” flow of neural activity (i.e., activity propagating from the frontal cortex to sensory areas) is more predictive of consciousness than a “bottom-up” flow of activity. The prefrontal cortex is not the only candidate area. We cannot assume, however, that the presence of a similar structural morphology is evidence of a particular experience/capacity/mental state, unless there are other reasons to support that conclusion.
Significant research has been conducted into identifying the neural correlates of consciousness (NCCs)—”the minimal neural mechanisms that are together necessary and sufficient for experiencing any conscious percept” [
56,
57,
58]. The theory of NCCs aim to identify the neural structures/brain activity that corresponds with and is necessary to produce a particular experience. Every phenomenal, subjective state will have associated NCCs. Identification of NCCs involves the correlation of activity with verbal reporting by a subject. Given the requirement for non-behavioural indicators of consciousness, it has been suggested these techniques could be applied to identify consciousness in human brain organoids [
16]. Considerable work has been completed to develop indicators and techniques for measuring the presence of consciousness in adults [
59,
60], and to apply these in cases of minimal states of consciousness, for example those in persistent vegetative states (PVS) to identify the minimal neuronal criteria necessary for consciousness [
48,
61,
62]. We discuss one example below in some detail to demonstrate the challenge of establishing coherent indicators of consciousness in human brain organoids.
The Perturbational Complexity Index (PCI), used for the clinical assessment of brain-injured, unresponsive patients, for example PVS [
62,
63] and brain injured non-communicating patients [
61], has been proposed as a metric for measuring consciousness in human brain organoids and to develop indicators for (neural activity) consciousness in human brain organoids [
10,
11,
21]. The PCI is based upon Integrated Information Theory (IIT) of consciousness which defines and quantifies consciousness in terms of the amount of integrated information in a system [
64,
65]. On the IIT account, a system has subjective experience (phenomenal consciousness) to the extent that it can integrate information. Organisms can be graded from those with lower to higher degrees of information integration along a spectrum of subjective experience and consciousness can be generated independently of sensory processing, executive functions and motor behaviour [
60]. NCCs serve as empirical data for evaluating IIT’s predictions [
66]. Lavazza and Massimini propose to measure information integration by a human brain organoid in response to stimulus to develop an index for human brain consciousness [
11]. This would form a basis for comparison with cases of minimal states of consciousness. If a “future objective index could suggest that some human cerebral organoids have some (although very low) capacity for consciousness, such a primitive level of consciousness could allow them to experience forms and various degrees/grades (i.e. ranging from mild to severe) of suffering somewhat similar to those experienced by individuals left in a permanently unresponsive state” (p. 609) [
11]. A “mere glimmer of consciousness” could elicit cause for concern (p. 609) [
11].
Concerns that human brain organoids may be sentient based on neuroscientific accounts of neural correlates of consciousness and information integration, encounter several difficulties. Extrapolating evidence of consciousness in unresponsive patients and demonstrating a relationship between the experience of an organism and simultaneous neural activity in its brain or brain-equivalent is not straightforward. An important consideration is whether the proxies developed for humans (from embodied human brain development) can be extrapolated to human brain organoids. That the neural networks are firing and the structures are in place is not evidence of conscious activity in human brain organoids. Identifying precursors for experience is not evidence of that experience. Moreover, drawing on our earlier discussion of consciousness and moral status, identifying a minimal level of consciousness is not enough to motivate a moral concern. IIT unpacks subjective awareness as kind of proto-consciousness—defined as experience separated from cognition or mind, it is not the kind of consciousness described earlier as sentience or self-awareness [
67]. The IIT account reduces consciousness to the most basic neuronal activity in a cortical region upon stimulation—what might be called pre-conscious sensory stimulation without subsequent subjective awareness of the sensory input [
2]. Such accounts belie a reductionism of mental states to patterns of electrical firing and lack a sufficiently rich account of what it is for a being to be conscious to justify any particular moral response to a being that is conscious on these theories. Even if ITT were to detect consciousness in human brain organoids, the theory is inadequate for showing whether and how human brain organoids matter morally on a philosophical account.
In summary, there is no consensus about what indicators to look for in developing human brains as a basis for claiming that a human brain organoid has consciousness and merits moral consideration. At present, we have no evidence that human brain organoids are conscious—we have no evidence of markers of sentience or evidence of conscious awareness, nor evidence of what needs to be in place to establish the presence of consciousness and that entities that are conscious must have this. There is an evidential gap between seeing similarity in a structure to seeing evidence of consciousness in that structure, between evidence of the hardware required for consciousness and evidence of consciousness arising from that hardware.
5. Consciousness, Moral Status and Human Brain Organoids: Challenges for Developing Regulations
In
Section 3, we evaluated concerns that human brain organoids may attain consciousness and based on this attainment may be owed a duty of care. We concluded that at present, claims by researchers of human brain organoids’ sentience and attributions of moral status based on these subjective states and attributions of personhood are overstated and unwarranted. In
Section 4, we reviewed approaches to identifying evidence of, and measuring consciousness in, human brain organoids. We identified an evidential gap from the discussion of evidence of consciousness in organoids to the presence of consciousness in human brain organoids as well as an explanatory gap that the presence of any such consciousness would matter morally. In this section, we bring these claims together to show how these evidential and explanatory gaps pose challenges for developing regulations for research on brain organoids based on the capacity for consciousness. Instead, we argue a more plausible approach to regulation is via obtaining a better understanding of human brain organoids as models and for what they are a proxy. This will allow for defeasible regulations, i.e., regulations that can be refined in light of further scientific or ethical research.
We have shown that we do not have evidence to support a claim of phenomenal consciousness in human brain organoids; thus, no reason to treat human brain organoids as conscious beings. Here, we present an epistemic and not an ontological argument—that we lack evidence to support a claim of consciousness—and do not make a claim about whether organoids are conscious now nor whether they will become so in the future. Brain organoids are living, (mostly) human cells’ structures that demonstrate surprising levels of organisation, and as models for brain and disease development demonstrate that complexity and organisation are important for showing how the brain works. As such, in their current state of development, regulations for handling tissue samples are appropriate and we should not unreasonably restrict research on human brain organoids based on concerns about consciousness.
As research develops, we may need to review our claim that we lack the evidence to support a claim of consciousness in human brain organoids. Some researchers have stated that if developed further, neural organoids have the potential to offer much more, to even “unlock” what makes us human [
68]. In the future, if the research on brain organoids leads to organoids that can be successfully developed well beyond their current level of complexity, we could come to have reason to consider their interests. For example, if they might develop and become integrated in a network of interconnections with sensory organs, stimuli and behavioural responses in a way that might suggest they could be phenomenally conscious, that they could experience pain, such that there may be “something it is like” to be a human brain organoid. Organoids have been generated with rudimentary sensory structures such as optic cups [
69]. However, it is still implausible that brain organoids would develop or come to be networked in a way that would provide evidence of consciousness in the sense of agency and self-awareness; even with such scientific advances, there would be no reason to view these more developed organoids as moral “selves”. In this scenario, whilst it might be the case that human brain organoids could be considered to have some of the features that support or indicate consciousness, it would be still contested what they might be like. Experience of a human brain organoid is likely to be very different from that of a pre-term infant, human, animal and not directly comparable.
Our conclusion concerning the appropriate regulation owed to human brain organoids in their present development is consistent with conclusions drawn by the majority of researchers who propose that in their current state of development the likelihood that human brain organoids possess consciousness is extremely low [
2,
7,
9,
21] and endorse regulations for handling tissue samples as appropriate for regulating their research [
7].
Whilst there is consensus on what regulations might apply for non-conscious human brain organoids, is there consensus based on consciousness? With a lack of “physical evidence of consciousness” and recognition of the epistemic and explanatory gaps identified in our paper, how do we develop plausible regulation for research involving emerging and more complex human brain organoids. The difficulties involved in developing regulation for human brain organoids is demonstrated by both the stalled attempts to reach agreement on defining consciousness in human brain organoids and the formulation of the appropriate regulation with respect to consciousness in human brain organoids [
2]. As Koplin and Savulescu note, not much progress has been made identifying the form that restrictions on the basis of consciousness should take [
7].
In recognition of our epistemic uncertainty—that we cannot rule out the possibility that brain organoids in the future may have the capacity to develop sentience and our inability to recognise and identify consciousness in human brain organoids—some urge adopting a precautionary approach to evidence of consciousness in human brain organoids [
7,
9,
16,
49]. Given “the onrushing ethical dilemma” identified earlier in this paper [
20], in the absence of evidence we should urge extra caution and we should not treat brain organoids as mere biological material if they could plausibly be conscious. Koplin and Savulescu argue “if we are unsure whether a particular being is conscious, we should not treat them as if they lack moral status, but instead err on the side of generosity and treat them as if they have at least partial moral status” (p. 762) [
7]. To formulate benchmarks for the regulation of research with potentially conscious human brain organoids, Koplin and Savulescu propose comparison with foetal brain development, which prior to 20 week lacks consciousness; thus, this acts as a proxy for the onset of phenomenal consciousness [
15,
70]. Some researchers have proposed the adoption and modification of research guidelines for animals in research as the most appropriate for research involving sentient human brain organoids [
2,
7]. The onset of higher cognitive functioning is identified as a further moral threshold and, on some accounts, implies personhood, though not all [
7]. Knowledge of how and when sentience begins, however, is subject to great uncertainty. Birch and Browning suggest that in contrast to foetal brain development we should look “directly for markers of sentience in organoids” in NCCs and argue “if an organoid contains structures or mechanisms that any serious and credible theory of human NCCs posits to be sufficient for conscious experience, we should take proportionate measures to regulate research on that organoid” (p. 57) [
16]. Birch and Browning note their proposal “sets the evidential bar for taking precautions at an intentionally low level” (p. 57) [
16].
Given the novel development of human brain organoids, reliance on developmental embryonic thresholds as parallels to serve as benchmarks for regulations have been criticised. Research with synthetic human entities with embryolike features (SHEEFs) shows that organoid development may not replicate developmental stages and may skip some steps in embryogenesis—the problem of bypass [
13]. This shows the inappropriateness of adopting the 14 day rule used to regulate research using conventional embryos and presuming embryonic development models may miss the development of consciousness in organoids [
13]. Instead of tying research limits to stages of canonical embryogenesis, Aach et al. argue limits should be based as directly as possible on the appearance of features or capacities that are associated with emergence of moral status: the appearance of neural substrates and functionality required for the experience of pain (p. 8) which would be immune from the problem of bypass [
13]. The criticism of conventional embryonic development resonates with recent claims that consciousness in other species may develop along different paths than just mammalian, including recent work on the development of consciousness in cephalopods [
71]. This raises the question, could human brain organoids realise a different developmental pathway? Consciousness in human brain organoids, if it were to develop, might likewise develop upon a different path and be different from the development of consciousness in humans, raising further epistemic challenges to identifying markers of consciousness. Though how that would develop independent of human and non-human embodiment is unclear. Given the ways that human brain organoids are unlike in vivo human brains raises the issue that we might attribute consciousness to a human brain organoid when it is not there, or miss consciousness in human brain organoids when it is there. Whilst the research guidelines for use of animals in research might be appropriate for research involving human–non-human chimeras, they may not be appropriate for disembodied, or novel-developed sentient human brain organoids.
Rather than making unwarranted moral assumptions about consciousness and adopting a precautionary approach when developing regulations around the use of brain organoids in research, we argue that research ethics would be better served by focusing attention on human brain organoids as models qua model and not as models qua human beings. To inform more reflective consideration about what limits should be placed on research, we “need to be aware of the similarities and differences between the model and the system which is being modelled. Once we have a better understanding of the model we can then have more reflective consideration about what limits should be placed on research” (p. 52) [
21]. This will allow for the formulation of defeasible regulations, ones that can be responsive to, and capable of being refined considering changes in scientific findings and ethical research. Appropriate regulation is based on and responds to changes in available evidence.
6. Conclusions
As models for brain and disease development, human brain organoids demonstrate that complexity and organisation are important for understanding how the brain works. Human brain organoids are extraordinary and extremely useful, with the potential to model human brain development and disease, for example, to test the toxicity of drugs and provide new therapies. However, there is currently a lack of evidence to support a claim of consciousness and to treat human brain organoids as conscious beings. Therefore, we should not unreasonably restrict research on human brain organoids. On the other hand, we should exercise epistemic humility as there are gaps in current knowledge that present challenges for developing defeasible regulations on the use of human brain organoids. As the scientific and ethical research develops over time, regulations can be refined. Research on human brain organoids may illuminate understanding of what is morally significant about consciousness for humans, other animals and potentially for human brain organoids.
Clarification of these issues requires sustained discussion of the moral significance of consciousness, the interrelation between theories of the nature of consciousness and empirical approaches to the assessment of consciousness, and the development of robust indicators of consciousness in human brain organoids. By bringing the philosophical and scientific perspectives into conversation together, we have shown that for philosophers the exploration opens up questions about what would be required in brain structures for evidence of consciousness (awareness) and, for neuroscientists, it opens up questions about what needs to be established about an entity’s experience to be the basis for moral consideration. Continued multidisciplinary collaboration and deep engagement involving biologists, philosophers, neuroscientists, cognitive scientists, bioethicists and legal scholars is needed to answer these questions and to ethically guide this important and emerging area of research.