1. Introduction
Laboratory- and pilot-scale studies have shown that nanotechnology has great potential in many sectors of the oil and gas industry, including enhanced oil recovery (EOR) [
1,
2]. Nanofluids can be used to improve the water injection process by altering the wettability of the porous medium, reducing the interfacial tension between the oil and the injected fluid, diverting the flow to unswept areas, or preventing asphaltene precipitation [
2,
3,
4]. Currently, ensuring the stability of nanofluids is critical and a great challenge for many operations [
5]. The formulation of the nanofluid (particle type, concentration, dispersants, etc.) must ensure stability (dispersion, low particle agglomeration) and absence of chemical changes from the preparation to flow in the reservoir [
6].
A nanofluid is a colloidal dispersion of nanoparticles (solid phase) in a base fluid (liquid phase). Its stability is determined by the nature of the interactions between the particles. Because the surface-to-volume ratio of the particles is so high, short-range forces, such as van der Waals attraction, and surface forces govern every interaction [
7]. Due to their Brownian motion, nanoparticles that come into contact with one another tend to stay together due to attractive forces, unless a longer-range repulsive force acts to keep the particles apart. Classical DLVO theory solely takes electrostatic repulsion into account, while the system may also be subject to steric repulsion and solvation forces [
8,
9].
Electrostatic repulsion is the result of the interaction between the electrical double layers surrounding the nanoparticles. When a charged surface is exposed to a liquid, a structure known as the electrical double layer appears on its surface (
Figure 1a). The first layer, called the Stern layer, is composed of counterions adsorbed onto the surface due to chemical interactions. The second layer is composed of ions attracted to the surface charge via Coulomb forces, thus electrically screening the first layer. This second layer is loosely associated with the surface and is therefore called the diffuse layer. With increasing distance from the nanoparticle, the high concentration of counterions within the diffuse layer progressively decreases until equilibrium is reached with the ion concentration in the bulk of the solvent. When the separation of the charged nanoparticles becomes less than twice the double-layer extension, the electrical double layers begin to overlap and repulsion occurs as the individual double layers can no longer expand without restriction [
10]. The length of the electric double layer
is called the Debye length. A larger nanoparticle surface charge and a longer Debye length lead to an increased stability of the nanoparticles in aqueous solution (
Figure 1b) [
11,
12].
However, the Debye length decreases as the ionic strength increases due to the high concentration of ions adsorbed on the particle surface and the compression of the layer (
Figure 1c) [
13]. The counterions in electrolytes have a screening effect, which reduces the electrostatic repulsion by offsetting the surface charges. The type of salt and its divalence are key parameters for the stability of the nanofluids: multivalent salts have a stronger shielding effect than monovalent salts. For example, nanoparticles in contact with seawater are always more unstable than
brine because it contains monovalent and multivalent salts (
,
,
, …).
Although silica nanoparticles have been extensively studied and their efficacy in EOR processes is well established [
14,
15,
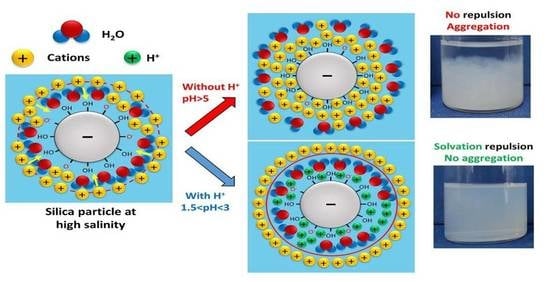
16], the applications of hydrophilic silica nanoparticles are constrained because of the ease with which they aggregate due to their highly energetic hydrophilic surfaces [
17]. Moreover, the reservoir conditions of high temperature and high salinity (ionic strength) favor nanofluid instability. Although Brownian motion is independent of the nature of the particle, it depends on the size of the particles and the viscosity of the base fluid. The motion becomes faster at higher temperature, favoring collisions between particles, and at high salinity, the repulsive forces are not strong enough to prevent aggregation. For successful EOR application, the long-term stability of nanoparticles under harsh reservoir conditions is essential.
One solution to increase the stability of nanofluids at reservoir conditions is to compensate for the loss of repulsive forces due to electrolytes in the solution by increasing electrostatic and steric repulsions through the modification of the pH of the base fluid. The protonation/deprotonation capacity of a nanoparticle’s surface is a crucial factor in the charge transfer between the particle and the solvent in an aqueous solution. The relative basicity or acidity of the solvent to the particle dictates the direction of proton transfer [
18,
19]. For silica nanoparticles, there are two protonation reactions:
For each system, there is a pH value at which the potential at the slipping plane (close to the Stern layer), called the zeta potential (ZP), which surrounds the particle, takes a value of zero. This pH is known as the isoelectric point (IEP) and is measured by electrophoretic light scattering [
19]. Consequently, to prepare electrostatically repelling suspensions, pH values away from the IEP are required. The IEP of hydrophilic silica is between 2 and 3.5 [
9,
20,
21,
22,
23]. Above the IEP, the zeta potential is negative, and as the pH increases towards 8, it decreases rapidly as
groups appear on the particle surface. As the pH increases from 8 to 12, the changes in zeta potential are less pronounced due to the large amount of ions present in the solution. The zeta potential of the suspension in these pH ranges varied between −30 and −70 mV, which is low enough to electrostatically stabilize the systems in the absence of electrolytes.
For this particular system of hydrophilic silica, some authors [
9,
20,
22,
24,
25] have noticed an abnormal stability at low pH values close to the IEP, which contrasts with that predicted by the classical DLVO theory [
26]. This means that there is another short-range repulsive force that is not accounted for in the theory. This force appears as a consequence of the particle hydration due to the ability of silanol groups (
) to form hydrogen bonds with water [
27]. The hydration force, which acts as a potent short-range repulsive force between two polar surfaces separated by a thin layer of water, diminishes nearly exponentially at decay lengths of around 1 nm [
28]. This repulsion can be considerable for hydrophilic nanoparticles in the presence of electrolytes [
29,
30].
The effectiveness of silica nanofluids for EOR applications relies on their dispersibility and ability to resist agglomeration during transport through the reservoir for long periods of time at extreme salinities (>10,000 ppm) and high temperatures (up to 150 °C) [
5]. Studies have shown that silica nanoparticles are extremely unstable in seawater and concentrated ionic solutions containing divalent ions, such as
,
, and
[
9,
23]. Recently, Sofla et al. proposed a theory based on “
protection”, which consists of the addition of
to stabilize silica nanoparticles in seawater at 25 °C [
9]. The results of this study demonstrate that the size of the nanoparticles in seawater directly relates to their concentration, which is inverse to the
content.
In this article, we investigated the stability of the silica nanofluid at different concentrations at reservoir conditions (high salinity and high temperature) as a function of base fluid pH. We compared the evolution of nanoparticle size and turbidity over time for monovalent and mixed ionic solutions as a function of time.
4. Final Remarks
This study was focused on investigating the possibility of stabilizing silica nanofluids at reservoir conditions, i.e., high salinity and high temperature. In the absence of any surfactant or polymer, van der Waals attraction outweighs the electrical double layer, causing silica nanoparticles to aggregate, thus leading to unstable suspensions.
A simple solution that was considered and tested was to adjust the pH of the base fluid. However, increasing the pH to basic values to increase the negative charge of the silica particles and thus maximize the electrostatic repulsive force does not work at high ionic strengths. The results showed that working at an acidic pH close to IEP intensifies the hydration repulsion between the silica particles and stabilizes them even in seawater at 80 °C. The concentration of nanoparticles is another important parameter to take into account: the higher the concentration, the less stable the nanofluid will be over time.
The results presented here define the conditions at which silica nanofluids are stable and can be injected in a reservoir as an enhanced oil recovery agent.