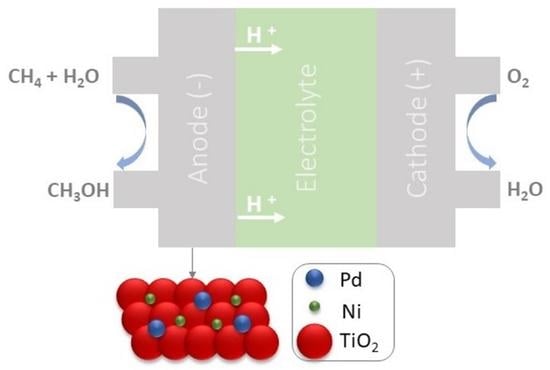
PdxNiy/TiO2 Electrocatalysts for Converting Methane to Methanol in An Electrolytic Polymeric Reactor—Fuel Cell Type (PER-FC)
Abstract
:1. Introduction
2. Results
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lange, J.-P.; Sushkevich, V.; Knorpp, A.; van Bokhoven, J.A. Methane-to-Methanol via Chemical Looping: Economic Potential and Guidance for Future Research. Ind. Eng. Chem. Res. 2019, 58, 8674–8680. [Google Scholar] [CrossRef]
- Blanco, H.; Nijs, W.; Ruf, J.; Faaij, A. Potential of Power-to-Methane in the EU energy transition to a low carbon system using cost optimization. Appl. Energy 2018, 232, 323–340. [Google Scholar] [CrossRef]
- Jang, J.; Shen, K.; Morales-Guio, C.G. Electrochemical Direct Partial Oxidation of Methane to Methanol. Joule 2019, 3, 2589–2593. [Google Scholar] [CrossRef]
- de Souza, R.; Florio, D.; Antolini, E.; Neto, A.O. Partial Methane Oxidation in Fuel Cell-Type Reactors for Co-Generation of Energy and Chemicals: A Short Review. Catalysts 2022, 12, 217. [Google Scholar] [CrossRef]
- Álvarez, M.; Marín, P.; Ordóñez, S. Direct oxidation of methane to methanol over Cu-zeolites at mild conditions. Mol. Catal. 2020, 487, 110886. [Google Scholar] [CrossRef]
- Tomkins, P.; Ranocchiari, M.; van Bokhoven, J. Direct Conversion of Methane to Methanol under Mild Conditions over Cu-Zeolites and beyond. Acc. Chem. Res. 2017, 50, 418–425. [Google Scholar] [CrossRef]
- Zhu, J.; Sushkevich, V.; Knorpp, A.; Newton, M.; Mizuno, S.; Wakihara, T.; Okubo, T.; Liu, Z.; van Bokhoven, J. Cu-Erionite Zeolite Achieves High Yield in Direct Oxidation of Methane to Methanol by Isothermal Chemical Looping. Chem. Mater. 2020, 32, 1448–1453. [Google Scholar] [CrossRef] [Green Version]
- Shavi, R.; Hiremath, V.; Seo, J. Radical-initiated oxidative conversion of methane to methanol over metallic iron and copper catalysts. Mol. Catal. 2018, 445, 232–239. [Google Scholar] [CrossRef]
- Xie, S.; Lin, S.; Zhang, Q.; Tian, Z.; Wang, Y. Selective electrocatalytic conversion of methane to fuels and chemicals. J. Energy Chem. 2018, 27, 1629–1636. [Google Scholar] [CrossRef] [Green Version]
- Nimkar, S.; Mewada, R.; Rosen, M. Exergy and exergoeconomic analyses of thermally coupled reactors for methanol synthesis. Int. J. Hydrogen Energy 2017, 42, 28113–28127. [Google Scholar] [CrossRef]
- Tomita, A.; Nakajima, J.; Hibino, T. Direct Oxidation of Methane to Methanol at Low Temperature and Pressure in an Electrochemical Fuel Cell. Angew. Chem. Int. Ed. 2008, 47, 1462–1464. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.; Santos, M.; Godoi, C.; Neto, A.O.; De Souza, R.F.B. Obtaining C2 and C3 Products from Methane Using Pd/C as Anode in a Solid Fuel Cell-type Electrolyte Reactor. ChemCatChem 2020, 12, 4517–4521. [Google Scholar] [CrossRef]
- Santos, M.; Nunes, L.; Silva, L.; Ramos, A.; Fonseca, F.; de Souza, R.; Neto, A. Direct Alkaline Anion Exchange Membrane Fuel Cell to Converting Methane into Methanol. ChemistrySelect 2019, 4, 11430–11434. [Google Scholar] [CrossRef]
- Santos, M.; Godoi, C.; Kang, H.; de Souza, R.; Ramos, A.; Antolini, E.; Neto, A. Effect of Ni content in PdNi/C anode catalysts on power and methanol co-generation in alkaline direct methane fuel cell type. J. Colloid Interface Sci. 2020, 578, 390–401. [Google Scholar] [CrossRef]
- Coelho, J.; Filho, N.; Gutierrez, I.; Godoi, C.; Gomes, P.; Zambiazi, P.; de Souza, R.; Neto, A. Methane-to-methanol conversion and power co-generation on palladium: Nickel supported on antimony tin oxide catalysts in a polymeric electrolyte reactor-fuel cell (PER-FC). Res. Chem. Intermed. 2022, 48, 5155–5168. [Google Scholar] [CrossRef]
- de Moura Souza, F.; de Souza, R.; Batista, B.; Santos, M.; Fonseca, F.; Neto, A.; Nandenha, J. Methane activation at low temperature in an acidic electrolyte using PdAu/C, PdCu/C, and PdTiO2/C electrocatalysts for PEMFC. Res. Chem. Intermed. 2020, 46, 2481–2496. [Google Scholar] [CrossRef]
- Mateos-Pedrero, C.; González-Carrazán, S.; Soria, M.; Ruíz, P. Effect of the nature of TiO2 support over the performances of Rh/TiO2 catalysts in the partial oxidation of methane. Catal. Today 2013, 203, 158–162. [Google Scholar] [CrossRef]
- Qu, W.; Wang, Z.; Sui, X.; Gu, D. An efficient antimony doped tin oxide and carbon nanotubes hybrid support of Pd catalyst for formic acid electrooxidation. Int. J. Hydrogen Energy 2014, 39, 5678–5688. [Google Scholar] [CrossRef]
- De Souza, R.; Neto, É.; Calegaro, M.; Santos, E.; Martinho, H.S.; dos Santos, M.C. Ethanol Electro-oxidation on Pt/C Electrocatalysts: An “In Situ” Raman Spectroelectrochemical Study. Electrocatalysis 2011, 2, 28–34. [Google Scholar] [CrossRef]
- Obradović, M.; Stančić, Z.; Lačnjevac, U.; Radmilović, V.; Gavrilović-Wohlmuther, A.; Radmilović, V.; Gojković, S. Electrochemical oxidation of ethanol on palladium-nickel nanocatalyst in alkaline media. Appl. Catal. B Environ. 2016, 189, 110–118. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, X.; Liu, H.; Wang, C.; Liu, S.; Sun, P.; Wang, L.; Liu, Y.; TiO, H. Heterostructured TiO2/WO3 porous microspheres: Preparation, characterization and photocatalytic properties. Catal. Today 2013, 201, 195–202. [Google Scholar] [CrossRef]
- Jeong, H.; Park, K.; Han, D.; Park, H. High efficiency solar chemical conversion using electrochemically disordered titania nanotube arrays transplanted onto transparent conductive oxide electrodes. Appl. Catal. B Environ. 2018, 226, 194–201. [Google Scholar] [CrossRef]
- Muniz-Miranda, M.; Zoppi, A.; Muniz-Miranda, F.; Calisi, N.; Nanoparticles, P.O. Characterization and Catalytic Activity Evaluation. Coatings 2020, 10, 207. [Google Scholar] [CrossRef] [Green Version]
- Mironova-Ulmane, N.; Kuzmin, A.; Steins, I.; Grabis, J.; Sildos, I.; Pärs, M. Raman scattering in nanosized nickel oxide NiO. J. Phys. Conf. Ser. 2007, 93, 012039. [Google Scholar] [CrossRef]
- Nandenha, J.; Piasentin, R.; Silva, L.; Fontes, E.; Neto, A.; de Souza, R. Partial oxidation of methane and generation of electricity using a PEMFC. Ionics 2019, 25, 5077–5082. [Google Scholar] [CrossRef]
- Beckingham, B.; Lynd, N.; Miller, D. Monitoring multicomponent transport using in situ ATR FTIR spectroscopy. J. Membr. Sci. 2018, 550, 348–356. [Google Scholar] [CrossRef] [Green Version]
- Fang, X.; Wang, L.; Shen, P.; Cui, G.; Bianchini, C. An in situ Fourier transform infrared spectroelectrochemical study on ethanol electrooxidation on Pd in alkaline solution. J. Power Sources 2010, 195, 1375–1378. [Google Scholar] [CrossRef]
- Fontes, E.; Piasentin, R.; Ayoub, J.; da Silva, J.; Assumpção, M.; Spinacé, E.; Neto, A.; de Souza, R. Electrochemical and in situ ATR-FTIR studies of ethanol electro-oxidation in alkaline medium using PtRh/C electrocatalysts. Mater. Renew. Sustain. Energy 2015, 4, 3. [Google Scholar] [CrossRef]
- Godoi, C.; Santos, M.; Silva, A.; Tagomori, T.; Ramos, A.; de Souza, R.; Neto, A. Methane conversion to higher value-added product and energy co-generation using anodes OF PdCu/C in a solid electrolyte reactor: Alkaline fuel cell type monitored by differential mass spectroscopy. Res. Chem. Intermed. 2021, 47, 743–757. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coelho, J.F.; Gutierrez, I.M.; Filho, N.G.P.; Zambiazi, P.J.; Neto, A.O.; de Souza, R.F.B. PdxNiy/TiO2 Electrocatalysts for Converting Methane to Methanol in An Electrolytic Polymeric Reactor—Fuel Cell Type (PER-FC). Methane 2023, 2, 137-147. https://doi.org/10.3390/methane2020011
Coelho JF, Gutierrez IM, Filho NGP, Zambiazi PJ, Neto AO, de Souza RFB. PdxNiy/TiO2 Electrocatalysts for Converting Methane to Methanol in An Electrolytic Polymeric Reactor—Fuel Cell Type (PER-FC). Methane. 2023; 2(2):137-147. https://doi.org/10.3390/methane2020011
Chicago/Turabian StyleCoelho, Jéssica F., Isabely M. Gutierrez, Nivaldo G. P. Filho, Priscilla J. Zambiazi, Almir O. Neto, and Rodrigo F. B. de Souza. 2023. "PdxNiy/TiO2 Electrocatalysts for Converting Methane to Methanol in An Electrolytic Polymeric Reactor—Fuel Cell Type (PER-FC)" Methane 2, no. 2: 137-147. https://doi.org/10.3390/methane2020011