A Multi-Faceted Approach to Quantifying Recovery of Stream Phytobenthos Following Acute Herbicide Incidents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Flume Design
2.2. Biofilm Functioning (Activity Measurements)
2.3. Biomass Measurements
2.4. Bacterial Abundance
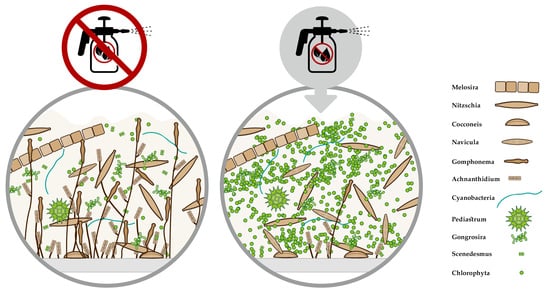
2.5. Algae and Cyanobacterial Species Composition
2.6. Data Analyses
3. Results
3.1. Short-Term Responses of Herbicides on Phytobenthos
3.1.1. Areal Biomass
3.1.2. Metabolic Responses to Herbicide Treatments
3.2. Longer-Term Responses in Biofilms 4.5 Days Post-Herbicide Removal
3.2.1. Biomass of Autotrophs and Heterotrophs
3.2.2. Enumeration of Bacterial Heterotrophs
3.2.3. Taxonomic Composition of Autotrophs within Biofilms
3.2.4. Taxonomic Analysis of Pennate Diatom Fraction, Based on Cleaned Valves in Phytobenthic Biofilms
3.2.5. Trophic Diatom Index in Relation to Herbicide Application
4. Discussion
4.1. Impacts of Chlorotoluron on Phytobenthic Biofilms
4.2. Impacts of a GBH and GBHC on Phytobenthic Biofilms
4.3. Changes in Species Composition of Phytobenthos
4.4. Implications for Ecological Assessment
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

| Diatom Guild | Control | Chlorotoluron | Glyphosate | Glyphosate and Chlorotoluron |
|---|---|---|---|---|
| Adnate/Short-Stalked | ||||
| Total biovolume × 106 [µm3 cm−2]: | 23 | 6 | 4 | 2 |
| Relative abundance %: | 19.7 | 4.1 | 2.5 | 4.1 |
| Erect | ||||
| Total biovolume × 106 [µm3 cm−2]: | 33 | 16 | 0 | 0 |
| Relative abundance %: | 28.2 | 10.1 | 0.0 | 0.0 |
| Motile | ||||
| Total biovolume × 106 [µm3 cm−2]: | 42 | 55 | 79 | 36 |
| Relative abundance %: | 35.6 | 35.0 | 51.8 | 78.5 |
| Filaments | ||||
| Total biovolume × 106 [µm3 cm−2]: | 20 | 80 | 69 | 8 |
| Relative abundance %: | 16.5 | 50.7 | 45.7 | 17.4 |
| Estimated Volume | Control | Chlorotoluron | Glyphosate | Glyphosate and Chlorotoluron | |
|---|---|---|---|---|---|
| Total number of cells × 106 [cells cm−2] | 0.67 | 0.55 | 1.30 | 1.05 | |
| Total biovolume × 106 [µm3 cm−2] | 157.94 | 218.43 | 292.75 | 129.67 | |
| [µm3] | ×106 [µm3 cm−2] | ×106 [µm3 cm−2] | ×106 [µm3 cm−2] | ×106 [µm3 cm−2] | |
| Desmodesmus sp. | 46 | 0.66 | 0.11 | 7.37 | 1.76 |
| Gongrosira (Colony) | 2356 | 3.19 | 0.34 | 0.83 | 0.19 |
| Green Flagelate | 248 | 0.30 | 0.00 | 3.67 | 7.15 |
| unid. Chlorophyta (spherical) (large) | 1150 | 1.38 | 12.41 | 36.31 | 17.92 |
| unid. Chlorophyta (spherical) (medium) | 74 | 11.03 | 8.38 | 24.03 | 17.47 |
| unid. Chlorophyta in pairs | 74 | 4.41 | 3.71 | 10.24 | 9.88 |
| Pediastrum sp. | 141 | 0.40 | 0.88 | 3.50 | 1.06 |
| Scenedesmussp. (groups of 4) (large) | 670 | 6.43 | 25.70 | 13.39 | 6.43 |
| Scenedesmus sp. (groups of 4) (medium) | 46 | 3.09 | 2.21 | 10.29 | 12.35 |
| Scenedesmus sp. (single) (large) | 670 | 3.21 | 3.21 | 24.90 | 3.21 |
| Scenedesmus sp. (single) (medium) | 46 | 1.27 | 1.10 | 2.68 | 2.70 |
References
- 2000/60/EC; Water Framework Directive: Maastricht, The Netherlands, 2000.
- Kelly, M.G.; Juggins, S.; Guthrie, R.; Pritchard, S.; Jamieson, J.; Rippey, B.; Hirst, H.; Yallop, M.L. Assessment of ecological status in UK rivers using diatoms. Freshw. Biol. 2008, 53, 403–422. [Google Scholar]
- Kelly, M. Data rich, information poor? Phytobenthos assessment and the Water Framework Directive. Eur. J. Phycol. 2013, 48, 437–450. [Google Scholar] [CrossRef]
- Juggins, S.; Kelly, M.; Allott, T.; Kelly-Quinn, M.; Monteith, D. A Water Framework Directive-compatible metric for assessing acidification in UK and Irish rivers using diatoms. Sci. Total Environ. 2016, 568, 671–678. [Google Scholar] [CrossRef] [Green Version]
- Poikane, S.; Kelly, M.; Cantonati, M. Benthic algal assessment of ecological status in European lakes and rivers: Challenges and opportunities. Sci. Total Environ. 2016, 568, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.; Bennion, H.; Burgess, A.; Ellis, J.; Juggins, S.; Guthrie, R.; Jamieson, J.; Adriaenssens, V.; Yallop, M. Uncertainty in ecological status assessments of lakes and rivers using diatoms. Hydrobiologia 2009, 633, 5–15. [Google Scholar] [CrossRef]
- Stoate, C.; Boatman, N.D.; Borralho, R.J.; Carvalho, C.R.; de Snoo, G.R.; Eden, P. Ecological impacts of arable intensification in Europe. J. Environ. Manag. 2001, 63, 337–365. [Google Scholar] [CrossRef]
- Wood, T.M. Herbicide Use in the Management of Roadside Vegetation, Western Oregon, 1999–2000: Effects on the Water Quality of nearby Streams; Report 01-4065; US Department of the Interior, US Geological Survey: Reston, VA, USA, 2001. [CrossRef]
- Hanke, I.; Wittmer, I.; Bischofberger, S.; Stamm, C.; Singer, H. Relevance of urban glyphosate use for surface water quality. Chemosphere 2010, 81, 422–429. [Google Scholar] [CrossRef]
- Coupe, R.H.; Kalkhoff, S.J.; Capel, P.D.; Gregoire, C. Fate and transport of glyphosate and aminomethylphosphonic acid in surface waters of agricultural basins. Pest Manag. Sci. 2012, 68, 16–30. [Google Scholar] [CrossRef]
- Lupi, L.; Bedmar, F.; Puricelli, M.; Marino, D.; Aparicio, V.C.; Wunderlin, D.; Miglioranza, K.S. Glyphosate runoff and its occurrence in rainwater and subsurface soil in the nearby area of agricultural fields in Argentina. Chemosphere 2019, 225, 906–914. [Google Scholar] [CrossRef]
- Debenest, T.; Silvestre, J.; Coste, M.; Pinelli, E. Effects of Pesticides on Freshwater Diatoms. In Reviews of Environmental Contamination and Toxicology; Springer: New York, NY, USA, 2010; pp. 87–103. [Google Scholar]
- Pesce, S.; Bouchez, A.; Montuelle, B. Effects of organic herbicides on phototrophic microbial communities in freshwater ecosystems. Rev. Environ. Contam. Toxicol. 2011, 214, 87–124. [Google Scholar] [CrossRef]
- Roubeix, V.; Mazzella, N.; Méchin, B.; Coste, M.; Delmas, F. Impact of the herbicide metolachlor on river periphytic diatoms: Experimental comparison of descriptors at different biological organization levels. Ann. De Limnol. Int. J. Limnol. 2011, 47, 239–249. [Google Scholar] [CrossRef] [Green Version]
- Segner, H.; Schmitt-Jansen, M.; Sabater, S. Assessing the impact of multiple stressors on aquatic biota: The receptor's side matters. Environ. Sci. Technol. 2014, 48, 7690–7696. [Google Scholar] [CrossRef]
- Begon, M.; Harper, J.L.; Townsend, C.R. Ecology: Individuals, Populations and Communities, 3rd ed.; Blackwell Science: Oxford, UK, 1999; ISBN 0632038012. [Google Scholar]
- Guasch, H.; Lehmann, V.; van Beusekom, B.; Sabater, S.; Admiraal, W. Influence of phosphate on the response of periphyton to atrazine exposure. Arch. Environ. Contam. Toxicol. 2007, 52, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Guasch, H.; Ivorra, N.; Lehmann, V.; Paulsson, M.; Real, M.; Sabater, S. Community composition and sensitivity of periphyton to atrazine in flowing waters: The role of environmental factors. J. Appl. Phycol. 1998, 10, 203–213. [Google Scholar] [CrossRef] [Green Version]
- Schmitt-Jansen, M.; Altenburger, R. Toxic effects of isoproturon on periphyton communities—A microcosm study. Estuar. Coast. Shelf Sci. 2005, 62, 539–545. [Google Scholar] [CrossRef]
- Larras, F.; Bouchez, A.; Rimet, F.; Montuelle, B. Using bioassays and species sensitivity distributions to assess herbicide toxicity towards benthic diatoms. PLoS ONE 2012, 7, e44458. [Google Scholar] [CrossRef] [Green Version]
- Pohlon, E.; Marxsen, J.; Küsel, K. Pioneering bacterial and algal communities and potential extracellular enzyme activities of stream biofilms. FEMS Microbiol. Ecol. 2010, 71, 364–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biggs, B.J.; Stevenson, R.J.; Lowe, R.L. A habitat matrix conceptual model for stream periphyton. Arch. Fur Hydrobiol. 1998, 143, 21–56. [Google Scholar] [CrossRef]
- Sabater, S.; Vilalta, E.; Gaudes, A.; Guasch, H.; Muñoz, I.; Romaní, A. Ecological implications of mass growth of benthic cyanobacteria in rivers. Aquat. Microb. Ecol. 2003, 32, 175–184. [Google Scholar] [CrossRef] [Green Version]
- Brasell, K.A.; Heath, M.W.; Ryan, K.G.; Wood, S.A. Successional change in microbial communities of benthic Phormidium-dominated biofilms. Microb. Ecol. 2015, 69, 254–266. [Google Scholar] [CrossRef]
- Paule, A.; Roubeix, V.; Lauga, B.; Duran, R.; Delmas, F.; Paul, E.; Rols, J.L. Changes in tolerance to herbicide toxicity throughout development stages of phototrophic biofilms. Aquat. Toxicol. 2013, 144–145, 310–321. [Google Scholar] [CrossRef] [Green Version]
- Yallop, M.L.; Kelly, M.G. From pattern to process: Understanding stream phytobenthic assemblages and implications for determining “ecological status”. Nova Hedwig. Beih. 2006, 130, 357–372. [Google Scholar]
- Sabio, Y.; García, C.A.; Vera, M.S.; Vinocur, A.; Graziano, M.; Miranda, C.; Pizarro, H.N. Rethinking the term “glyphosate effect” through the evaluation of different glyphosate-based herbicide effects over aquatic microbial communities. Environ. Pollut. 2022, 292, 118382. [Google Scholar] [CrossRef]
- Brovini, E.M.; Cardoso, S.J.; Quadra, G.R.; Vilas-Boas, J.A.; Paranaíba, J.R.; Pereira, R.d.O.; Mendonça, R.F. Glyphosate concentrations in global freshwaters: Are aquatic organisms at risk? Environ. Sci. Pollut. Res. Int. 2021, 28, 60635–60648. [Google Scholar] [CrossRef] [PubMed]
- Székács, A.; Darvas, B. Re-registration Challenges of Glyphosate in the European Union. Front. Environ. Sci. 2018, 6, 78. [Google Scholar] [CrossRef] [Green Version]
- van Bruggen, A.H.C.; He, M.M.; Shin, K.; Mai, V.; Jeong, K.C.; Finckh, M.R.; Morris, J.G. Environmental and health effects of the herbicide glyphosate. Sci. Total Environ. 2018, 616–617, 255–268. [Google Scholar] [CrossRef]
- Agathokleous, E. European Union’s imminent ban on glyphosate: Hormesis should be considered in new chemical screening and selection. J. For. Res. 2022, 33, 1103–1107. [Google Scholar] [CrossRef]
- Hébert, M.-P.; Fugère, V.; Gonzalez, A. The overlooked impact of rising glyphosate use on phosphorus loading in agricultural watersheds. Front. Ecol. Environ. 2019, 17, 48–56. [Google Scholar] [CrossRef] [Green Version]
- Marín-Benito, J.M.; Carpio, M.J.; Mamy, L.; Andrades, M.S.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S. Field measurement and modelling of chlorotoluron and flufenacet persistence in unamended and amended soils. Sci. Total Environ. 2020, 725, 138374. [Google Scholar] [CrossRef]
- Beckie, H.J.; Flower, K.C.; Ashworth, M.B. Farming without Glyphosate? Plants 2020, 9, 96. [Google Scholar] [CrossRef] [Green Version]
- Carabias-Martínez, R. Determination of herbicides, including thermally labile phenylureas, by solid-phase microextraction and gas chromatography–mass spectrometry. J. Chromatogr. 2003, 1002, 1–12. [Google Scholar] [CrossRef]
- Annett, R.; Habibi, H.R.; Hontela, A. Impact of glyphosate and glyphosate-based herbicides on the freshwater environment. J. Appl. Toxicol. 2014, 34, 458–479. [Google Scholar] [CrossRef]
- Ledger, M.E.; Harris, R.M.L.; Armitage, P.D.; Milner, A.M. Realism of model ecosystems: An evaluation of physicochemistry and macroinvertebrate assemblages in artificial streams. Hydrobiologia 2009, 617, 91–99. [Google Scholar] [CrossRef]
- Sabater, S.; Guasch, H.; Ricart, M.; Romaní, A.; Vidal, G.; Klünder, C.; Schmitt-Jansen, M. Monitoring the effect of chemicals on biological communities. The biofilm as an interface. Anal. Bioanal. Chem. 2007, 387, 1425–1434. [Google Scholar] [CrossRef]
- Gold, C.; Feurtet-Mazel, A.; Coste, M.; Boudou, A. Effects of cadmium stress on periphytic diatom communities in indoor artificial streams. Freshw. Biol. 2003, 48, 316–328. [Google Scholar] [CrossRef]
- Cedergreen, N.; Streibig, J.C. The toxicity of herbicides to non-target aquatic plants and algae: Assessment of predictive factors and hazard. Pest Manag. Sci. 2005, 61, 1152–1160. [Google Scholar] [CrossRef]
- Ma, J.Y.; Wang, S.F.; Wang, P.W.; Ma, L.J.; Chen, X.L.; Xu, R.F. Toxicity assessment of 40 herbicides to the green alga Raphidocelis subcapitata. Ecotoxicol. Environ. Saf. 2006, 63, 456–462. [Google Scholar] [CrossRef]
- Cairns, J.; Niederlehner, B.R. (Eds.) Ecological Toxicity Testing. Scale, Complexity, and Relevance; Lewis Publishers: Boca Raton, FL, USA, 1995; ISBN 978-0873715997. [Google Scholar]
- Jassby, A.D.; Platt, T. Mathematical formulation of the relationship between photosynthesis and light for phytoplankton. Limnol. Oceanogr. 1976, 21, 540–547. [Google Scholar] [CrossRef] [Green Version]
- Lorenzen, C.J. Determination of Chlorophyll and Pheo-Pigments: Spectrophotometric Equations. Limnol. Oceanogr. 1967, 12, 343–346. [Google Scholar] [CrossRef]
- Porter, K.G.; Feig, Y.S. The use of DAPI for identifying and counting aquatic microflora. Limnol. Oceanogr. 1980, 25, 943–948. [Google Scholar] [CrossRef]
- Sherr, E.B.; Sherr, B.F. Preservation and storage of samples for enumeration of heterotrophic protists. Handbook of methods in aquatic microbial ecology. In Handbook of Methods in Aquatic Microbial Ecology; Kemp, P.F., Sherr, B.F., Sherr, E.B., Cole, J.J., Eds.; CRC Press: Boca Raton, FL, USA; London, UK; New York, NY, USA, 1993; pp. 207–212. ISBN 9780203752746. [Google Scholar]
- Carpenter, A.E.; Jones, T.R.; Lamprecht, M.R.; Clarke, C.; Kang, H.; Friman, O.; Guertin, D.A.; Chang, J.H.; Lindquist, R.A.; Moffat, J.; et al. CellProfiler: Image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006, 7, R100. [Google Scholar] [CrossRef] [PubMed]
- Vokes, M.S.; Carpenter, A.E. Using CellProfiler for Automatic Identification and Measurement of Biological Objects in Images. Curr. Protoc. Mol. Biol. 2008, 82, 14.17.1–14.17.12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brierley, B.; Carvalho, L.; Davies, S.; Krokowski, J. Guidance on the Quantitative Analysis of Phytoplankton in Freshwater Samples; Report to SNIFFER (Project WFD80); Edinburgh, Scotland, 2007. [Google Scholar]
- Cox, E.J. Identification of Freshwater Diatoms from Live Material; Chapman and Hall: London, UK, 1996. [Google Scholar]
- Gutowski, A.; Foerster, J. Benthische Algen ohne Diatomeen und Characeen. Bestimmungshilfe; LANUV-Arbeitsblatt: Recklinghausen, Germany, 2009; Volume 9. [Google Scholar]
- John, D.M. (Ed.) The Freshwater Algal Flora of the British Isles: An Identification Guide to Freshwater and Terrestrial Algae, 2nd ed.; Cambridge University Press: Cambridge, UK, 2011; ISBN 978-1108478007. [Google Scholar]
- Guiry, M.D. AlgaeBase. World-Wide Electronic Publication. Available online: http://www.algaebase.org (accessed on 16 October 2022).
- Bock, C.; Krienitz, L.; Pröschold, T. Taxonomic reassessment of the genus Chlorella (Trebouxiophyceae) using molecular signatures (barcodes), including description of seven new species. FOTTEA 2011, 11, 293–312. [Google Scholar] [CrossRef] [Green Version]
- Leliaert, F.; Smith, D.R.; Moreau, H.; Herron, M.D.; Verbruggen, H.; Delwiche, C.F.; de Clerck, O. Phylogeny and Molecular Evolution of the Green Algae. Crit. Rev. Plant Sci. 2012, 31, 1–46. [Google Scholar] [CrossRef] [Green Version]
- Neustupa, J.; Němcová, Y.; Veselá, J.; Steinová, J.; Škaloud, P. Parachloroidium gen. nov. (Trebouxiophyceae, Chlorophyta), a novel genus of coccoid green algae from subaerial corticolous biofilms. Phycologia 2013, 52, 411–421. [Google Scholar] [CrossRef] [Green Version]
- Hillebrand, H.; Dürselen, C.-D.; Kirschtel, D.; Pollingher, U.; Zohary, T. Biovolume Calculation for Pelagic and Benthic Microalgae. J. Phycol. 1999, 35, 403–424. [Google Scholar] [CrossRef]
- OECD. Test No. 201: Freshwater Alga and Cyanobacteria, Growth Inhibition Test: OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2006; ISBN 9789264069923. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; Hoagland, K.D.; Siegfried, B.D. Uptake and Bioconcentration of Atrazine by Selected Freshwater Algae. Environ. Toxicol. Chem. 1998, 17, 1085–1090. [Google Scholar] [CrossRef]
- Moro, C.V.; Bricheux, G.; Portelli, C.; Bohatier, J. Comparative effects of the herbicides chlortoluron and mesotrione on freshwater microalgae. Environ. Toxicol. Chem. 2012, 31, 778–786. [Google Scholar] [CrossRef]
- Anton, F.A.; Ariz, M.; Alia, M. Ecotoxic effects of four herbicides (glyphosate, alachlor, chlortoluron and isoproturon) on the algae Chlorella pyrenoidosa Chick. Sci. Total Environ. 1993, 134, 845–851. [Google Scholar] [CrossRef]
- Guasch, H.; Admiraal, W.; Sabater, S. Contrasting effects of organic and inorganic toxicants on freshwater periphyton. Aquat. Toxicol. 2003, 64, 165–175. [Google Scholar] [CrossRef]
- Gerbersdorf, S.U.; Westrich, B.; Paterson, D.M. Microbial extracellular polymeric substances (EPS) in fresh water sediments. Microb. Ecol. 2009, 58, 334–349. [Google Scholar] [CrossRef] [PubMed]
- Rimet, F.; Bouchez, A. Use of diatom life-forms and ecological guilds to assess pesticide contamination in rivers: Lotic mesocosm approaches. Ecol. Indic. 2011, 11, 489–499. [Google Scholar] [CrossRef] [Green Version]
- Pérès, F.; Florin, D.; Grollier, T.; Feurtet-Mazel, A.; Coste, M.; Ribeyre, F.; Ricard, M.; Boudou, A. Effects of the phenylurea herbicide isoproturon on periphytic diatom communities in freshwater indoor microcosms. Environ. Pollut. 1996, 94, 141–152. [Google Scholar] [CrossRef]
- Gomes, M.P.; Le Manac'h, S.G.; Hénault-Ethier, L.; Labrecque, M.; Lucotte, M.; Juneau, P. Glyphosate-Dependent Inhibition of Photosynthesis in Willow. Front. Plant Sci. 2017, 8, 207. [Google Scholar] [CrossRef] [Green Version]
- Tsui, M.T.; Chu, L.M. Aquatic toxicity of glyphosate-based formulations: Comparison between different organisms and the effects of environmental factors. Chemosphere 2003, 52, 1189–1197. [Google Scholar] [CrossRef]
- Romero, D.M.; Ríos de Molina, M.C.; Juárez, A.B. Oxidative stress induced by a commercial glyphosate formulation in a tolerant strain of Chlorella kessleri. Ecotoxicol. Envion. Saf. 2011, 74, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Sasal, M.C.; Wilson, M.G.; Sione, S.M.; Beghetto, S.M.; Gabioud, E.A.; Oszust, J.D.; Paravani, E.V.; Demonte, L.; Repetti, M.R.; Bedendo, D.J.; et al. Monitoring of glyphosate in surface water in the province of Entre Ríos. Participatory action research as a collaborative methodology. Rev. De Investig. Agropecu. 2017, 43, 195–205. [Google Scholar]
- Tajnaiová, L.; Vurm, R.; Kholomyeva, M.; Kobera, M.; Kočí, V. Determination of the Ecotoxicity of Herbicides Roundup® Classic Pro and Garlon New in Aquatic and Terrestrial Environments. Plants 2020, 9, 1203. [Google Scholar] [CrossRef]
- Nagy, K.; Tessema, R.A.; Budnik, L.T.; Ádám, B. Comparative cyto- and genotoxicity assessment of glyphosate and glyphosate-based herbicides in human peripheral white blood cells. Environ. Res. 2019, 179, 108851. [Google Scholar] [CrossRef]
- Lozano, V.L.; Vinocur, A.; Sabio y García, C.A.; Allende, L.; Cristos, D.S.; Rojas, D.; Wolansky, M.; Pizarro, H. Effects of glyphosate and 2,4-D mixture on freshwater phytoplankton and periphyton communities: A microcosms approach. Ecotoxicol. Environ. Saf. 2018, 148, 1010–1019. [Google Scholar] [CrossRef]
- Goldsborough, L.G.; Robinson, G.G.C. Pattern in wetlands. In Algal Ecology; Elsevier: Amsterdam, The Netherlands, 1996; pp. 77–109. ISBN 9780126684506. [Google Scholar]
- Singh, S.; Kumar, V.; Gill, J.P.K.; Datta, S.; Singh, S.; Dhaka, V.; Kapoor, D.; Wani, A.B.; Dhanjal, D.S.; Kumar, M.; et al. Herbicide Glyphosate: Toxicity and Microbial Degradation. Int. J. Environ. Res. Public Health 2020, 17, 7519. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, J.R.; Peterson, C.G. Emigration and immigration can be important determinants of benthic diatom assemblages in streams. Freshw. Biol. 1991, 26, 279–294. [Google Scholar] [CrossRef]
- Bothwell, M.L. Phosphorus–Limited Growth Dynamics of Lotic Periphytic Diatom Communities: Areal Biomass and Cellular Growth Rate Responses. Can. J. Fish. Aquat. Sci. 1989, 46, 1293–1301. [Google Scholar] [CrossRef]
- Peterson, C.G. Response of Benthic Algal Communities to Natural Physical Disturbance. In Algal Ecology; Elsevier: Amsterdam, The Netherlands, 1996; pp. 375–402. ISBN 9780126684506. [Google Scholar]
- Yallop, M.; Wang, Y.; Masuda, S.; Daniels, J.; Ockenden, A.; Masani, H.; Scott, T.B.; Xie, F.; Ryan, M.; Jones, C.; et al. Quantifying impacts of titanium dioxide nanoparticles on natural assemblages of riverine phytobenthos and phytoplankton in an outdoor setting. Sci. Total Environ. 2022, 831, 154616. [Google Scholar] [CrossRef]
- Hill, W.R.; Boston, H.L. Community development alters photosynthesis-irradiance relations in stream periphyton. Limnol. Oceanogr. 1991, 36, 1375–1389. [Google Scholar] [CrossRef]
- Wood, R.J.; Mitrovic, S.M.; Lim, R.P.; Kefford, B.J. How benthic diatoms within natural communities respond to eight common herbicides with different modes of action. Sci. Total Environ. 2016, 557–558, 636–643. [Google Scholar] [CrossRef]
- Vera, M.S.; Di Fiori, E.; Lagomarsino, L.; Sinistro, R.; Escaray, R.; Iummato, M.M.; Juárez, A.; Del Ríos de Molina, M.C.; Tell, G.; Pizarro, H. Direct and indirect effects of the glyphosate formulation Glifosato Atanor® on freshwater microbial communities. Ecotoxicology 2012, 21, 1805–1816. [Google Scholar] [CrossRef]
- Magbanua, F.S.; Townsend, C.R.; Hageman, K.J.; Lange, K.; Lear, G.; Lewis, G.D.; Matthaei, C.D. Understanding the combined influence of fine sediment and glyphosate herbicide on stream periphyton communities. Water Res. 2013, 47, 5110–5120. [Google Scholar] [CrossRef]
- Sylwestrzak, Z.; Zgrundo, A.; Pniewski, F. Ecotoxicological Studies on the Effect of Roundup® (Glyphosate Formulation) on Marine Benthic Microalgae. Int. J. Environ. Res. Public Health 2021, 18, 884. [Google Scholar] [CrossRef]
- Perez, G.L.; Torremorell, A.; Mugni, H.; Rodriguez, P.; Vera, M.S.; Do Nascimento, M.; Allende, L.; Bustingorry, J.; Escaray, R.; Ferraro, M.; et al. Effects of the herbicide roundup on freshwater microbial communities: A mesocosm study. Ecol. Appl. 2007, 17, 2310–2322. [Google Scholar] [CrossRef] [Green Version]
- Vera, M.S.; Lagomarsino, L.; Sylvester, M.; Pérez, G.L.; Rodríguez, P.; Mugni, H.; Sinistro, R.; Ferraro, M.; Bonetto, C.; Zagarese, H.; et al. New evidences of Roundup (glyphosate formulation) impact on the periphyton community and the water quality of freshwater ecosystems. Ecotoxicology 2010, 19, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Pizarro, H.; Vera, M.S.; Vinocur, A.; Pérez, G.; Ferraro, M.; Menéndez Helman, R.J.; Dos Santos Afonso, M. Glyphosate input modifies microbial community structure in clear and turbid freshwater systems. Environ. Sci. Pollut. Res. Int. 2016, 23, 5143–5153. [Google Scholar] [CrossRef] [PubMed]
- Sura, S.; Waiser, M.; Tumber, V.; Farenhorst, A. Effects of herbicide mixture on microbial communities in prairie wetland ecosystems: A whole wetland approach. Sci. Total Environ. 2012, 435–436, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Saxton, M.A.; Morrow, E.A.; Bourbonniere, R.A.; Wilhelm, S.W. Glyphosate influence on phytoplankton community structure in Lake Erie. J. Great Lakes Res. 2011, 37, 683–690. [Google Scholar] [CrossRef]
- Austin, A.P.; Harris, G.E.; Lucey, W.P. Impact of an Organophosphate Herbicide (Glyphosate) on Periphyton Communities Developed in Experimental Streams. Bull. Environ. Contam. Toxicol. 1991, 47, 29–35. [Google Scholar] [CrossRef]
- Bowes, M.J.; Neal, C.; Jarvie, H.P.; Smith, J.T.; Davies, H.N. Predicting phosphorus concentrations in British rivers resulting from the introduction of improved phosphorus removal from sewage effluent. Sci. Total Environ. 2010, 408, 4239–4250. [Google Scholar] [CrossRef]
- Bowes, M.J.; Smith, J.T.; Neal, C.; Leach, D.V.; Scarlett, P.M.; Wickham, H.D.; Harman, S.A.; Armstrong, L.K.; Davy-Bowker, J.; Haft, M.; et al. Changes in water quality of the River Frome (UK) from 1965 to 2009: Is phosphorus mitigation finally working? Sci. Total Environ. 2011, 409, 3418–3430. [Google Scholar] [CrossRef]
- Roubeix, V.; Mazzella, N.; Delmas, F.; Coste, M. In situ evaluation of herbicide effects on the composition of river periphytic diatom communities in a region of intensive agriculture. Vie Et Milieu Life Environ. 2010, 60, 233–241. [Google Scholar]
- Rier, S.T.; Stevenson, R.J. Response of Periphytic Algae to Gradients in Nitrogen and Phosphorus in Streamside Mesocosms. Hydrobiologia 2006, 561, 131–147. [Google Scholar] [CrossRef]
- EFSA Panel on Plant Protection Products and their Residues. Scientific Opinion on the development of specific protection goal options for environmental risk assessment of pesticides, in particular in relation to the revision of the Guidance Documents on Aquatic and Terrestrial Ecotoxicology (SANCO/3268/2001 and SA). EFSA J. 2010, 8, 1821. [Google Scholar] [CrossRef] [Green Version]
- Kelly, M. Simplicity is the ultimate sophistication: Building capacity to meet the challenges of the Water Framework Directive. Ecol. Indic. 2014, 36, 519–523. [Google Scholar] [CrossRef]
- DeLorenzo, M.E.; Scott, G.I.; Ross, P.E. Toxicity of pesticides to aquatic microorganisms: A review. Environ. Toxicol. Chem. 2001, 20, 84. [Google Scholar] [CrossRef] [PubMed]
- Potapova, M.; Charles, D.F. Diatom metrics for monitoring eutrophication in rivers of the United States. Ecol. Indic. 2007, 7, 48–70. [Google Scholar] [CrossRef]






| Treatment | Chl. a (µg cm−2) | DM (mg cm−2) | AFDM (mg cm−2) |
|---|---|---|---|
| CON | 2.98 ± 0.53 | 3.53 ± 0.71 | 1.05 ± 0.14 |
| CLT | 2.25 ± 0.39 | 3.46 ± 0.95 | 1.12 ± 0.21 |
| GBH | 0.73 ± 0.04 * | 1.94 ± 1.19 * | 0.81 ± 0.21 |
| GBHC | 0.67 ± 0.06 * | 1.67 ± 0.48 * | 0.77 ± 0.09 * |
| Treatment | Chl. a (µg cm−2) | DM (mg cm−2) | AFDM (mg cm−2) |
|---|---|---|---|
| CON | 2.24 ± 0.58 | 2.65 ± 0.58 | 0.89 ± 0.10 |
| CLT | 2.71 ± 0.79 | 2.48 ± 0.55 | 0.85 ± 0.11 |
| GBH | 2.38 ± 0.56 | 1.60 ± 0.35 | 0.78 ± 0.07 |
| GBHC | 1.22 ± 0.34 | 1.19 ± 0.42 | 0.70 ± 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosenkranz, H.; Kelly, M.G.; Anesio, A.M.; Yallop, M.L. A Multi-Faceted Approach to Quantifying Recovery of Stream Phytobenthos Following Acute Herbicide Incidents. Phycology 2023, 3, 25-46. https://doi.org/10.3390/phycology3010003
Rosenkranz H, Kelly MG, Anesio AM, Yallop ML. A Multi-Faceted Approach to Quantifying Recovery of Stream Phytobenthos Following Acute Herbicide Incidents. Phycology. 2023; 3(1):25-46. https://doi.org/10.3390/phycology3010003
Chicago/Turabian StyleRosenkranz, Helen, Martyn G. Kelly, Alexandre M. Anesio, and Marian L. Yallop. 2023. "A Multi-Faceted Approach to Quantifying Recovery of Stream Phytobenthos Following Acute Herbicide Incidents" Phycology 3, no. 1: 25-46. https://doi.org/10.3390/phycology3010003