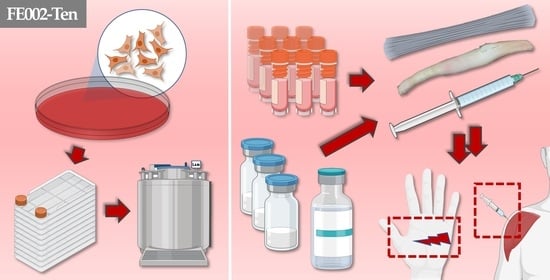
Primary Progenitor Tenocytes: Cytotherapeutics and Cell-Free Derivatives
Definition
:1. Introduction
2. Biological Material Procurement and Cell Source Establishment Methodology: FE002 Primary Progenitor Tenocytes for Clinical Applications
3. Primary Progenitor Tenocytes for Allogeneic Tissue Engineering Applications and for Cytotherapies
3.1. Primary Progenitor Tenocyte Manufacturing Processes
- Parental cell banks (PCB), at early in vitro passage levels;
- Master cell banks (MCB), at intermediate in vitro passage levels;
- Working cell banks (WCB), at in vitro passage levels appropriate for clinical use;
- End of production cell banks (EOPCB), at in vitro passage levels beyond those appropriate for clinical use.
- Retrieval and initiation of the cryopreserved starting materials;
- Qualitative and quantitative assessments of the initiated cellular suspension;
- Serial seeding of an appropriate in vitro cell culture system using predefined technical specifications;
- Incubation of the cell culture system with periodical culture medium exchanges and monitoring;
- Endpoint enzymatic harvest of the expanded and confluent cell population;
- Qualitative and quantitative assessments of the harvested cell suspension;
- Cellular bulk formulation and dilution in a cryoprotectant solution and serial dispensing in storage vessels;
- Controlled freezing of the conditioned cellular bulk lot;
- Cryogenic storage of the conditioned cellular bulk lot.
- Cellular morphology and adherent behavior in microscopy following recovery;
- Cell type identification by genetic, biochemical, or immunological means;
- Cell type karyotype establishment;
- Cell type in vitro lifespan determination;
- Testing for bacterial and fungal contamination;
- Testing for mycobacteria and mycoplasmas;
- Electron microscopy for elucidation of cellular structures;
- Testing for extraneous agents in cell cultures;
- Testing for viruses and for retroviruses;
- Safety/toxicity testing in small animals or in chicken eggs;
- Tumorigenicity assays (i.e., in vitro, in vivo).
- Use of consistent technical specifications, targets, and acceptance criteria;
- Use of thoroughly qualified contact-process consumables, materials, and reagents;
- Use of 10% v/v fetal bovine serum (FBS) as a cell proliferation medium supplement;
- Use of a humidified 5% CO2 incubation atmosphere at 37 °C, with 21% O2 or 2% O2;
- Use of FBS-based and dimethyl sulfoxide-based cryopreservation medium;
- Use of constant-rate freezing devices prior to cell bank lot cryogenic storage.
3.2. Primary Progenitor Tenocyte In Vitro Characterization Data
- Impacts of hypoxia on key and critical cellular attributes [28];
- Cellular proliferation stimulation potency in co-cultures with irradiated primary adult tenocytes [20];
- Cellular genetic stability at in vitro passages levels of 3–12 [20];
- Proteomic contents [28];
- Applicability in bio-printed tissue engineering [30];
- Cellular survival at 4 °C in hydrogels and hydrogel stability, maintenance of attachment and proliferation capacities [9];
- Cellular survival after extrusion through clinical administration systems [9];
- Absence of tumorigenic behavior in vitro (i.e., soft agar transformation assays) [22].
3.3. Primary Progenitor Tenocyte Preclinical Safety Evidence
3.4. Regulatory Considerations and Limitations for Tissue Engineering Products Containing Viable Primary Progenitor Tenocytes
3.5. Economic Considerations around Cell Therapy Production and Applicability in Modern Healthcare Systems
4. Lyophilized Primary Progenitor Tenocyte Derivatives for Potent Functionalization of Hyaluronan-Based Hydrogels
4.1. Primary Progenitor Tenocyte Stabilized Derivative Manufacturing Processes
- Descriptive and photographic controls of the lyophilizate vial lots;
- pH value determination of the reconstituted lyophilizates;
- Osmolality determination of the reconstituted lyophilizates;
- Study of sample particulate population distribution (i.e., except in cell-free extracts);
- Determination of lyophilizate resuspension time;
- Determination of lyophilizate residual moisture levels.
4.2. Primary Progenitor Tenocyte Stabilized Derivative Characterization Data
- Lyophilizate total proteomic contents;
- Lyophilizate stability in storage over time;
- Lyophilizate intrinsic antioxidant activity;
- Lyophilizate cytocompatibility with primary patient cells;
- Hyaluronan-based hydrogel viscosity modulation function of the lyophilizates;
- Hyaluronan-based hydrogel viscosity modulation function of the lyophilizates in oxidative environments (e.g., H2O2 challenge assays);
- Complex hydrogel preparation injectability evaluation in vitro/ex vivo;
- Complex hydrogel preparation bioadhesivity evaluation in vitro/ex vivo;
- Complex hydrogel preparation tribology characterization in vitro/ex vivo.
4.3. Technical Development and Rationale for the Use of Stabilized Progenitor Tenocyte Derivatives as Hyaluronan-Based Hydrogel Stability Enhancement Agents
4.4. Important Logistical Advantages of Stabilized Progenitor Tenocyte Derivatives over Classical Cytotherapies
4.5. Regulatory Considerations for the Therapeutic Use of Stabilized Primary Progenitor Tenocyte Derivatives
5. Conclusions and Prospects
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATMP | advanced therapy medicinal product |
| 60Co | cobalt 60 irradiation source |
| CAM | chorioallantoic membrane model |
| cATMP | combined advanced therapy medicinal product |
| CD | cluster of differentiation |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DMSO | dimethyl sulfoxide |
| ECM | extracellular matrix |
| EOPCB | end of production cell bank |
| FBS | fetal bovine serum |
| FDA | US Food and Drug Administration |
| FE002-Cart | primary chondroprogenitor cell source |
| FE002-SK2 | primary dermal progenitor fibroblast cell source |
| FE002-Ten | primary progenitor tenocyte cell source |
| FITC | fluorescein isothiocyanate |
| GAG | glycosaminoglycan |
| GLP | good laboratory practices |
| GMP | good manufacturing practices |
| HA | hyaluronic acid |
| HLA | human leucocyte antigen |
| H2O2 | hydrogen peroxide |
| HPL | human platelet lysate |
| ITS | insulin-transferrin-selenium |
| kGy | kiloGray |
| MCB | master cell bank |
| MD | medical device |
| PCB | parental cell bank |
| PE | phycoerythrin |
| PMDA | Japanese Pharmaceuticals and Medical Devices Agency |
| PRP | platelet-rich plasma |
| TEAC | Trolox equivalent antioxidant capacity |
| TFDA | Taiwan Food and Drug Administration |
| TrSt | standardized transplant product |
| WCB | working cell bank |
References
- Vacanti, J.P.; Langer, R. Tissue engineering: The design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet 1999, 354, 32–34. [Google Scholar] [CrossRef] [PubMed]
- Ramezankhani, R.; Torabi, S.; Minaei, N.; Madani, H.; Rezaeiani, S.; Hassani, S.N.; Gee, A.P.; Dominici, M.; Silva, D.N.; Baharvand, H.; et al. Two decades of global progress in authorized advanced therapy medicinal products: An emerging revolution in therapeutic strategies. Front. Cell Dev. Biol. 2020, 8, 547653. [Google Scholar] [CrossRef]
- Laurent, A.; Rey, M.; Scaletta, C.; Abdel-Sayed, P.; Michetti, M.; Flahaut, M.; Raffoul, W.; de Buys Roessingh, A.; Hirt-Burri, N.; Applegate, L.A. Retrospectives on three decades of safe clinical experience with allogeneic dermal progenitor fibroblasts: High versatility in topical cytotherapeutic care. Pharmaceutics 2023, 15, 184. [Google Scholar] [CrossRef]
- Clayton, R.A.; Court-Brown, C.M. The epidemiology of musculoskeletal tendinous and ligamentous injuries. Injury 2008, 39, 1338–1344. [Google Scholar] [CrossRef]
- Costa-Almeida, R.; Calejo, I.; Gomes, M.E. Mesenchymal stem cells empowering tendon regenerative therapies. Int. J. Mol. Sci. 2019, 20, 3002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirghaderi, S.P.; Valizadeh, Z.; Shadman, K.; Lafosse, T.; Oryadi-Zanjani, L.; Yekaninejad, M.S.; Nabian, M.H. Cell therapy efficacy and safety in treating tendon disorders: A systemic review of clinical studies. J. Exp. Orthop. 2022, 9, 85. [Google Scholar] [CrossRef]
- Kaux, J.F.; Samson, A.; Crielaard, J.M. Hyaluronic acid and tendon lesions. Muscles Ligaments Tendons J. 2016, 5, 264–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abate, M.; Schiavone, C.; Salini, V. The use of hyaluronic acid after tendon surgery and in tendinopathies. BioMed Res. Int. 2014, 2014, 783632. [Google Scholar] [CrossRef] [Green Version]
- Grognuz, A.; Scaletta, C.; Farron, A.; Pioletti, D.P.; Raffoul, W.; Applegate, L.A. Stability enhancement using hyaluronic acid gels for delivery of human fetal progenitor tenocytes. Cell Med. 2016, 8, 87–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masiello, F.; Pati, I.; Veropalumbo, E.; Pupella, S.; Cruciani, M.; De Angelis, V. Ultrasound-guided injection of platelet-rich plasma for tendinopathies: A systematic review and meta-analysis. Blood Transfus. 2022; in press. [Google Scholar] [CrossRef]
- Ho, J.O.; Sawadkar, P.; Mudera, V. A review on the use of cell therapy in the treatment of tendon disease and injuries. J. Tissue Eng. 2014, 5, 2041731414549678. [Google Scholar] [CrossRef]
- Chen, X.; Song, X.H.; Yin, Z.; Zou, X.H.; Wang, L.L.; Hu, H.; Cao, T.; Zheng, M.; Ouyang, H.W. Stepwise differentiation of human embryonic stem cells promotes tendon regeneration by secreting fetal tendon matrix and differentiation factors. Stem Cells 2009, 27, 1276–1287. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, Y.; Liu, E.; Sun, Y.; Luo, Z.; Xu, Z.; Liu, W.; Zhong, L.; Lv, Y.; Wang, A.; et al. Human iPSC-derived neural crest stem cells promote tendon repair in a rat patellar tendon window defect model. Tissue Eng. Part A 2013, 19, 2439–2451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chong, A.K.; Ang, A.D.; Goh, J.C.; Hui, J.H.; Lim, A.Y.; Lee, E.H.; Lim, B.H. Bone marrow-derived mesenchymal stem cells influence early tendon-healing in a rabbit Achilles tendon model. J. Bone Jt. Surg. Am. 2007, 89, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Van den Boom, N.A.C.; Winters, M.; Haisma, H.J.; Moen, M.H. Efficacy of stem cell therapy for tendon disorders: A systematic review. Orthop. J. Sport. Med. 2020, 8, 2325967120915857. [Google Scholar] [CrossRef] [PubMed]
- Andriolo, L.; Altamura, S.A.; Reale, D.; Candrian, C.; Zaffagnini, S.; Filardo, G. Nonsurgical treatments of patellar tendinopathy: Multiple injections of platelet-rich plasma are a suitable option: A systematic review and meta-analysis. Am. J. Sport. Med. 2019, 47, 1001–1018. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.; Barrachina, L.; Ranera, B.; Remacha, A.R.; Moreno, B.; de Blas, I.; Sanz, A.; Vasquez, F.J.; Vitoria, A.; Junquera, C.; et al. Comparison of autologous bone marrow and adipose tissue derived mesenchymal stem cells, and platelet-rich plasma, for treating surgically induced lesions of the equine superficial digital flexor tendon. Vet. J. 2017, 224, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Van der Vlist, A.C.; Winters, M.; Weir, A.; Ardern, C.L.; Welton, N.J.; Caldwell, D.M.; Verhaar, J.A.; de Vos, R.J. Which treatment is most effective for patients with Achilles tendinopathy? A living systematic review with network meta-analysis of 29 randomised controlled trials. Br. J. Sport. Med. 2021, 55, 249–256. [Google Scholar] [CrossRef]
- Freedman, B.R.; Mooney, D.J.; Weber, E. Advances toward transformative therapies for tendon diseases. Sci. Transl. Med. 2022, 14, eabl8814. [Google Scholar] [CrossRef]
- Grognuz, A.; Scaletta, C.; Farron, A.; Raffoul, W.; Applegate, L.A. Human fetal progenitor tenocytes for regenerative medicine. Cell Transpl. 2016, 25, 463–479. [Google Scholar] [CrossRef] [Green Version]
- Laurent, A.; Hirt-Burri, N.; Scaletta, C.; Michetti, M.; de Buys Roessingh, A.S.; Raffoul, W.; Applegate, L.A. Holistic approach of Swiss fetal progenitor cell banking: Optimizing safe and sustainable substrates for regenerative medicine and biotechnology. Front. Bioeng. Biotechnol. 2020, 8, 557758. [Google Scholar] [CrossRef]
- Laurent, A.; Abdel-Sayed, P.; Grognuz, A.; Scaletta, C.; Hirt-Burri, N.; Michetti, M.; de Buys Roessingh, A.S.; Raffoul, W.; Kronen, P.; Nuss, K.; et al. Industrial development of standardized fetal progenitor cell therapy for tendon regenerative medicine: Preliminary safety in xenogeneic transplantation. Biomedicines 2021, 9, 380. [Google Scholar] [CrossRef]
- Zhang, K.; Na, T.; Wang, L.; Gao, Q.; Yin, W.; Wang, J.; Yuan, B.Z. Human diploid MRC-5 cells exhibit several critical properties of human umbilical cord-derived mesenchymal stem cells. Vaccine 2014, 32, 6820–6827. [Google Scholar] [CrossRef]
- Olshansky, S.J.; Hayflick, L. The role of the WI-38 cell strain in saving lives and reducing morbidity. AIMS Public Health 2017, 4, 127–138. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Points to Consider in the Characterization of Cell Lines Used to Produce Biologicals; FDA: Silver Spring, MD, USA, 1993.
- Aeberhard, P.A.; Grognuz, A.; Peneveyre, C.; McCallin, S.; Hirt-Burri, N.; Antons, J.; Pioletti, D.; Raffoul, W.; Applegate, L.A. Efficient decellularization of equine tendon with preserved biomechanical properties and cytocompatibility for human tendon surgery indications. Artif. Organs 2020, 44, E161–E171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laurent, A.; Porcello, A.; Jeannerat, A.; Peneveyre, C.; Coeur, A.; Abdel-Sayed, P.; Scaletta, C.; Michetti, M.; de Buys Roessingh, A.; Jordan, O.; et al. Lyophilized progenitor tenocyte extracts: Sterilizable cytotherapeutic derivatives with antioxidant properties and hyaluronan hydrogel functionalization effects. Antioxidants 2023, 12, 163. [Google Scholar] [CrossRef] [PubMed]
- Jeannerat, A.; Peneveyre, C.; Armand, F.; Chiappe, D.; Hamelin, R.; Scaletta, C.; Hirt-Burri, N.; de Buys Roessingh, A.; Raffoul, W.; Applegate, L.A.; et al. Hypoxic incubation conditions for optimized manufacture of tenocyte-based active pharmaceutical ingredients of homologous standardized transplant products in tendon regenerative medicine. Cells 2021, 10, 2872. [Google Scholar] [CrossRef]
- Laurent, A.; Porcello, A.; Fernandez, P.G.; Jeannerat, A.; Peneveyre, C.; Abdel-Sayed, P.; Scaletta, C.; Hirt-Burri, N.; Michetti, M.; de Buys Roessingh, A.; et al. Combination of hyaluronan and lyophilized progenitor cell derivatives: Stabilization of functional hydrogel products for therapeutic management of tendinous tissue disorders. Pharmaceutics 2021, 13, 2196. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chansoria, P.; Delrot, P.; Angelidakis, E.; Rizzo, R.; Rütsche, D.; Applegate, L.A.; Loterie, D.; Zenobi-Wong, M. Filamented light (flight) biofabrication of highly aligned tissue-engineered constructs. Adv. Mater. 2022, 34, 2204301. [Google Scholar] [CrossRef]
- European Directorate for the Quality of Medicines & Healthcare. European Directorate for the Quality of Medicines & Healthcare. European Committee (Partial Agreement) on organ transplantation (CD-P-TO). In Guide to the Quality and Safety of Tissues and Cells for Human Application, 4th ed.; EDQM: Strasbourg, France, 2019; ISBN 978-92-871-8945-5. [Google Scholar]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [Green Version]
- Hopkins, C.; Fu, S.C.; Chua, E.; Hu, X.; Rolf, C.; Mattila, V.M.; Qin, L.; Yung, P.S.; Chan, K.M. Critical review on the socio-economic impact of tendinopathy. Asia Pac. J. Sport. Med. Arthrosc. Rehabil. Technol. 2016, 4, 9–20. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Maffulli, N. Tendon injury and tendinopathy: Healing and repair. J. Bone Jt. Surg. Am. 2005, 87, 187–202. [Google Scholar] [CrossRef] [Green Version]
- Fujikawa, K.; Ohtani, T.; Matsumoto, H.; Seedhom, B.B. Reconstruction of the extensor apparatus of the knee with the Leeds-Keio ligament. J. Bone Jt. Surg. Br. 1994, 76, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Chong, A.K.; Riboh, J.; Smith, R.L.; Lindsey, D.P.; Pham, H.M.; Chang, J. Flexor tendon tissue engineering: Acellularized and reseeded tendon constructs. Plast. Reconstr. Surg. 2009, 123, 1759–1766. [Google Scholar] [CrossRef]
- Lovati, A.B.; Bottagisio, M.; Moretti, M. Decellularized and engineered tendons as biological substitutes: A critical review. Stem Cells Int. 2016, 2016, 7276150. [Google Scholar] [CrossRef] [Green Version]
- Pridgen, B.C.; Woon, C.Y.; Kim, M.; Thorfinn, J.; Lindsey, D.; Pham, H.; Chang, J. Flexor tendon tissue engineering: Acellularization of human flexor tendons with preservation of biomechanical properties and biocompatibility. Tissue Eng. Part C Method. 2011, 17, 819–828. [Google Scholar] [CrossRef]
- Kryger, G.S.; Chong, A.K.; Costa, M.; Pham, H.; Bates, S.J.; Chang, J. A comparison of tenocytes and mesenchymal stem cells for use in flexor tendon tissue engineering. J. Hand Surg. Am. 2007, 32, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Larson, B.J.; Longaker, M.T.; Lorenz, H.P. Scarless fetal wound healing: A basic science review. Plast. Reconstr. Surg. 2010, 126, 1172–1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liau, L.L.; Hassan, M.N.F.B.; Tang, Y.L.; Ng, M.H.; Law, J.X. Feasibility of human platelet lysate as an alternative to foetal bovine serum for in vitro expansion of chondrocytes. Int. J. Mol. Sci. 2021, 22, 1269. [Google Scholar] [CrossRef] [PubMed]
- Philippe, V.; Laurent, A.; Hirt-Burri, N.; Abdel-Sayed, P.; Scaletta, C.; Schneebeli, V.; Michetti, M.; Brunet, J.F.; Applegate, L.A.; Martin, R. Retrospective analysis of autologous chondrocyte-based cytotherapy production for clinical use: GMP process-based manufacturing optimization in a Swiss university hospital. Cells 2022, 11, 1016. [Google Scholar] [CrossRef]
- Johnson, P.C.; Bertram, T.A.; Tawil, B.; Hellman, K.B. Hurdles in tissue engineering/regenerative medicine product commercialization: A survey of North American academia and industry. Tissue Eng. Part A 2011, 17, 5–15. [Google Scholar] [CrossRef]
- Pirnay, J.P.; Vanderkelen, A.; De Vos, D.; Draye, J.P.; Rose, T.; Ceulemans, C.; Ectors, N.; Huys, I.; Jennes, S.; Verbeken, G. Business oriented EU human cell and tissue product legislation will adversely impact Member States’ health care systems. Cell Tissue Bank 2013, 14, 525–560. [Google Scholar] [CrossRef]
- Pearce, K.F.; Hildebrandt, M.; Greinix, H.; Scheding, S.; Koehl, U.; Worel, N.; Apperley, J.; Edinger, M.; Hauser, A.; Mischak-Weissinger, E.; et al. Regulation of advanced therapy medicinal products in Europe and the role of academia. Cytotherapy 2014, 16, 289–297. [Google Scholar] [CrossRef] [Green Version]
- Evans, C.H.; Ghivizzani, S.C.; Robbins, P.D. Orthopaedic gene therapy: Twenty-five years on. JBJS Rev. 2021, 9, e20. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, R.; Chowdhury, C.R.; Arega, S.; Sen, P.; Ganguly, P.; Ganguly, N. CAR T cell therapy: A new era for cancer treatment (Review). Oncol. Rep. 2019, 42, 2183–2195. [Google Scholar] [CrossRef] [PubMed]
- Laurent, A.; Scaletta, C.; Abdel-Sayed, P.; Michetti, M.; Flahaut, M.; Simon, J.P.; Roessingh, A.B.; Raffoul, W.; Hirt-Burri, N.; Applegate, L.A. Optimized manufacture of lyophilized dermal fibroblasts for next-generation off-the-shelf progenitor biological bandages in topical post-burn regenerative medicine. Biomedicines 2021, 9, 1072. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Sayed, P.; Hirt-Burri, N.; de Buys Roessingh, A.; Raffoul, W.; Applegate, L.A. Evolution of biological bandages as first cover for burn patients. Adv. Wound Care 2019, 8, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Chemali, M.; Laurent, A.; Scaletta, C.; Waselle, L.; Simon, J.P.; Michetti, M.; Brunet, J.F.; Flahaut, M.; Hirt-Burri, N.; Raffoul, W.; et al. Burn center organization and cellular therapy integration: Managing risks and costs. J. Burn Care Res. 2021, 42, 911–924. [Google Scholar] [CrossRef]
- Gobet, R.; Raghunath, M.; Altermatt, S.; Meuli-Simmen, C.; Benathan, M.; Dietl, A.; Meuli, M. Efficacy of cultured epithelial autografts in pediatric burns and reconstructive surgery. Surgery 1997, 121, 654–661. [Google Scholar] [CrossRef]
- Oliva, F.; Marsilio, E.; Asparago, G.; Frizziero, A.; Berardi, A.C.; Maffulli, N. The impact of hyaluronic acid on tendon physiology and its clinical application in tendinopathies. Cells 2021, 10, 3081. [Google Scholar] [CrossRef]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef]
- Brown, M.B.; Jones, S.A. Hyaluronic acid: A unique topical vehicle for the localized delivery of drugs to the skin. J. Eur. Acad. Dermatol. Venereol. 2005, 19, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Dicker, K.T.; Gurski, L.A.; Pradhan-Bhatt, S.; Witt, R.L.; Farach-Carson, M.C.; Jia, X. Hyaluronan: A simple polysaccharide with diverse biological functions. Acta Biomater. 2014, 10, 1558–1570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huerta-Ángeles, G.; Nešporová, K.; Ambrožová, G.; Kubala, L.; Velebný, V. An effective translation: The development of hyaluronan-based medical products from the physicochemical, and preclinical aspects. Front. Bioeng. Biotechnol. 2018, 6, 62. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Li, Y.; Zhang, T.; Shi, M.; Song, X.; Yang, S.; Liu, H.; Zhang, M.; Cui, Q.; Li, Z. Hepatocyte growth factor-induced tendon stem cell conditioned medium promotes healing of injured Achilles tendon. Front. Cell Dev. Biol. 2021, 9, 654084. [Google Scholar] [CrossRef] [PubMed]
- Lu, V.; Tennyson, M.; Zhang, J.; Khan, W. Mesenchymal stem cell-derived extracellular vesicles in tendon and ligament repair—A systematic review of in vivo studies. Cells 2021, 10, 2553. [Google Scholar] [CrossRef]
- Song, K.; Jiang, T.; Pan, P.; Yao, Y.; Jiang, Q. Exosomes from tendon derived stem cells promote tendon repair through miR-144-3p-regulated tenocyte proliferation and migration. Stem Cell Res. Ther. 2022, 13, 80. [Google Scholar] [CrossRef]
- Xue, Z.; Chen, Z.; Wu, T.; Li, R.; Chen, C.; Liu, J.; Hou, H.; Zheng, X.; Wang, H. VEGFA-enriched exosomes from tendon-derived stem cells facilitate tenocyte differentiation, migration, and transition to a fibroblastic phenotype. BioMed Res. Int. 2022, 2022, 8537959. [Google Scholar] [CrossRef]
- Yao, Z.; Li, J.; Xiong, H.; Cui, H.; Ning, J.; Wang, S.; Ouyang, X.; Qian, Y.; Fan, C. MicroRNA engineered umbilical cord stem cell-derived exosomes direct tendon regeneration by mTOR signaling. J. Nanobiotechnol. 2021, 19, 169. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, H.; Cui, Q.; Han, P.; Yang, S.; Shi, M.; Zhang, T.; Zhang, Z.; Li, Z. Tendon stem cell-derived exosomes regulate inflammation and promote the high-quality healing of injured tendon. Stem Cell Res. Ther. 2020, 11, 402. [Google Scholar] [CrossRef]
- Lui, P.P.Y.; Leung, Y.T. Practical considerations for translating mesenchymal stromal cell-derived extracellular vesicles from bench to bed. Pharmaceutics 2022, 14, 1684. [Google Scholar] [CrossRef]
- Cottle, C.; Porter, A.P.; Lipat, A.; Turner-Lyles, C.; Nguyen, J.; Moll, G.; Chinnadurai, R. Impact of cryopreservation and freeze-thawing on therapeutic properties of mesenchymal stromal/stem cells and other common cellular therapeutics. Curr. Stem Cell Rep. 2022, 8, 72–92. [Google Scholar] [CrossRef] [PubMed]
- Gimona, M.; Brizzi, M.F.; Choo, A.B.H.; Dominici, M.; Davidson, S.M.; Grillari, J.; Hermann, D.M.; Hill, A.F.; de Kleijn, D.; Lai, R.C.; et al. Critical considerations for the development of potency tests for therapeutic applications of mesenchymal stromal cell-derived small extracellular vesicles. Cytotherapy 2021, 23, 373–380. [Google Scholar] [CrossRef]
- Sadeghi, S.; Tehrani, F.R.; Tahmasebi, S.; Shafiee, A.; Hashemi, S.M. Exosome engineering in cell therapy and drug delivery. Inflammopharmacology 2023, 31, 145–169. [Google Scholar] [CrossRef] [PubMed]
- Racchi, M.; Govoni, S.; Lucchelli, A.; Capone, L.; Giovagnoni, E. Insights into the definition of terms in European medical device regulation. Expert Rev. Med. Dev. 2016, 13, 907–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conrozier, T.; Mathieu, P.; Rinaudo, M. Mannitol preserves the viscoelastic properties of hyaluronic acid in an in vitro model of oxidative stress. Rheumatol. Ther. 2014, 1, 45–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schanté, C.E.; Zuber, G.; Herlin, C.; Vandamme, T.F. Chemical modifications of hyaluronic acid for the synthesis of derivatives for a broad range of biomedical applications. Carbohydr. Polym. 2011, 85, 469–489. [Google Scholar] [CrossRef]
- Schanté, C.; Zuber, G.; Herlin, C.; Vandamme, T.F. Synthesis of N-alanyl-hyaluronamide with high degree of substitution for enhanced resistance to hyaluronidase-mediated digestion. Carbohydr. Polym. 2011, 86, 747–752. [Google Scholar] [CrossRef]
- Aviv, M.; Halperin-Sternfeld, M.; Grigoriants, I.; Buzhansky, L.; Mironi-Harpaz, I.; Seliktar, D.; Einav, S.; Nevo, Z.; Adler-Abramovich, L. Improving the mechanical rigidity of hyaluronic acid by integration of a supramolecular peptide matrix. ACS Appl. Mater. Interfaces 2018, 10, 41883–41891. [Google Scholar] [CrossRef]
- Karel, S.; Sogorkova, J.; Hermannova, M.; Nesporova, K.; Marholdova, L.; Chmelickova, K.; Bednarova, L.; Flegel, M.; Drasar, P.; Velebny, V. Stabilization of hyaluronan-based materials by peptide conjugation and its use as a cell-seeded scaffold in tissue engineering. Carbohydr. Polym. 2018, 201, 300–307. [Google Scholar] [CrossRef]
- Porcello, A.; Gonzalez-Fernandez, P.; Jordan, O.; Allémann, E. Nanoforming hyaluronan-based thermoresponsive hydrogels: Optimized and tunable functionality in osteoarthritis management. Pharmaceutics 2022, 14, 659. [Google Scholar] [CrossRef]
- Zheng, Y.; Yang, J.; Liang, J.; Xu, X.; Cui, W.; Deng, L.; Zhang, H. Bioinspired hyaluronic acid/phosphorylcholine polymer with enhanced lubrication and anti-inflammation. Biomacromolecules 2019, 20, 4135–4142. [Google Scholar] [CrossRef] [PubMed]
- Amadio, P.C. Gliding resistance and modifications of gliding surface of tendon: Clinical perspectives. Hand Clin. 2013, 29, 159–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maudens, P.; Meyer, S.; Seemayer, S.A.; Jordan, O.; Allémann, E. Self-assembled thermoresponsive nanostructures of hyaluronic acid conjugates for osteoarthritis therapy. Nanoscale 2018, 10, 1845–1854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kogan, G.; Soltés, L.; Stern, R.; Schiller, J.; Mendichi, R. Hyaluronic acid: Its function and degradation in in vivo systems. Stud. Nat. Prod. Chem. 2008, 34, 789–882. [Google Scholar] [CrossRef]
- Fakhari, A.; Berkland, C. Applications and emerging trends of hyaluronic acid in tissue engineering, as a dermal filler and in osteoarthritis treatment. Acta Biomater. 2013, 9, 7081–7092. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.; Zhang, Y.; Ossipov, D. Enzymatic degradation of hyaluronan hydrogels with different capacity for in situ bio-mineralization. Biopolymers 2018, 109, e23090. [Google Scholar] [CrossRef]
- Rinaudo, M.; Lardy, B.; Grange, L.; Conrozier, T. Effect of mannitol on hyaluronic acid stability in two in vitro models of oxidative stress. Polymers 2014, 6, 1948–1957. [Google Scholar] [CrossRef] [Green Version]
- Remmele, R.L.; Krishnan, S.; Callahan, W.J. Development of stable lyophilized protein drug products. Curr. Pharm. Biotechnol. 2012, 13, 471–496. [Google Scholar] [CrossRef]
- Ó’Fágáin, C.; Colliton, K. Storage and lyophilization of pure proteins. Methods Mol. Biol. 2017, 1485, 159–190. [Google Scholar] [CrossRef]
- Fernandez-Moure, J.; Maisha, N.; Lavik, E.B.; Cannon, J.W. The chemistry of lyophilized blood products. Bioconj. Chem. 2018, 29, 2150–2160. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; DePaz, R.A.; Bee, J.S.; Marshall, T. Development of a stable lyophilized adeno-associated virus gene therapy formulation. Int. J. Pharm. 2021, 606, 120912. [Google Scholar] [CrossRef]
- Meneghel, J.; Kilbride, P.; Morris, G.J. Cryopreservation as a key element in the successful delivery of cell-based therapies—A review. Front. Med. 2020, 7, 592242. [Google Scholar] [CrossRef] [PubMed]
- Fehm, T.; Boehme, P.; Modak, N.; Talwar, V.; Kinscher, K. The clinical supply of cell and gene therapy drugs: Challenges ahead. Drug Discov. Today 2023, 28, 103421. [Google Scholar] [CrossRef] [PubMed]
- Naik, S.; Chougule, M.; Padhi, B.K.; Misra, A. Development of novel lyophilized mixed micelle amphotericin B formulation for treatment of systemic fungal infection. Curr. Drug Deliv. 2005, 2, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Nordberg, R.C.; Otarola, G.A.; Wang, D.; Hu, J.C.; Athanasiou, K.A. Navigating regulatory pathways for translation of biologic cartilage repair products. Sci. Transl. Med. 2022, 14, eabp8163. [Google Scholar] [CrossRef]




| Study Subject/Domain | Scope of Study Data/Investigated Parameters | References |
|---|---|---|
| 1. FE002-Ten Cell Source Establishment | Establishment of the FE002-Ten cell source in a cryopreserved multi-tiered biobank following a single controlled organ donation. | [21] |
| 2. FE002-Ten Cell Type In Vitro Characterization | Characterization of primary progenitor tenocyte attributes (e.g., cell population homogeneity and purity, genetic and phenotypic stability, proteomic contents, biological functions) 1. | [9,20,21,22,28] |
| 3. FE002-Ten Cell Type Biobanking & Manufacturing | Establishment of optimized and standardized in vitro primary progenitor tenocyte manufacturing workflows for the production of industrial scale cellular material lots. | [22,28] |
| 4. FE002-Ten Cell Type Preclinical Safety Characterization | Characterization of primary progenitor tenocyte safety (i.e., at clinically relevant passage levels 2) in vitro (e.g., genetic stability, tumorigenicity assays) and in vivo (e.g., CAM model, GLP study of cell implantation in rabbit tendons). | [20,22] |
| 5. FE002-Ten Cell Type Derivative Manufacturing, Lyophilization, and Sterilization | Establishment of biological material processing and purification workflows, for cell-derived and cell-free stabilized formulation obtention. Optimization of pharmaceutical processing (e.g., two-step lyophilization) for temperature stabilization of the cellular extracts. Optimization of the sterilization methodologies (e.g., submicron filtration, 60Co gamma irradiation) for conservation of cell-derived extract critical quality attributes and functional properties. | [27,29] |
| 6. FE002-Ten Cells or Derivatives: Study of Combination Product Prototypes | Translational characterization of primary progenitor tenocytes for tissue engineering applications (e.g., using injectable hydrogels, collagen scaffolds, artificial and biological tendon matrices). Translational characterization of hyaluronan hydrogel-based devices incorporating stabilized cellular derivatives. | [9,20,26,27,29,30] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laurent, A.; Scaletta, C.; Abdel-Sayed, P.; Raffoul, W.; Hirt-Burri, N.; Applegate, L.A. Primary Progenitor Tenocytes: Cytotherapeutics and Cell-Free Derivatives. Encyclopedia 2023, 3, 340-361. https://doi.org/10.3390/encyclopedia3010021
Laurent A, Scaletta C, Abdel-Sayed P, Raffoul W, Hirt-Burri N, Applegate LA. Primary Progenitor Tenocytes: Cytotherapeutics and Cell-Free Derivatives. Encyclopedia. 2023; 3(1):340-361. https://doi.org/10.3390/encyclopedia3010021
Chicago/Turabian StyleLaurent, Alexis, Corinne Scaletta, Philippe Abdel-Sayed, Wassim Raffoul, Nathalie Hirt-Burri, and Lee Ann Applegate. 2023. "Primary Progenitor Tenocytes: Cytotherapeutics and Cell-Free Derivatives" Encyclopedia 3, no. 1: 340-361. https://doi.org/10.3390/encyclopedia3010021