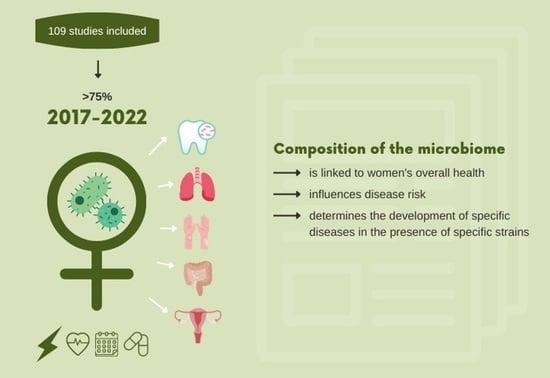
The Importance of the Microbiota in Shaping Women’s Health—The Current State of Knowledge
Abstract
:1. Introduction
- -
- The area having permanent colonization—skin, mucous membranes of the upper and lower respiratory tract, upper and lower gastrointestinal tract (especially oral cavity and large intestine), vagina;
- -
- The area having little transitional colonization—larynx, trachea, bronchi, lateral sinuses of the nose, the middle section of the gastrointestinal tract (esophagus, stomach, the upper part of the small intestine, urethra, cervix, conjunctiva;
- -
- The non-colonized area—bronchioles, alveolus, tears, blood, cerebrospinal fluid, urine, tissues, and tissue fluids [10].
2. Materials and Methods
2.1. Methodology Background
2.2. Review Procedure and Search Strategy
2.3. Sources Selection
3. Microbiota Distribution
3.1. Skin
3.2. Oral Cavity
3.3. The Gastrointestinal Tract
3.4. Respiratory Tract
3.5. Genitourinary System Respiratory Tract
3.6. Effects of Hormones on a Woman’s Microbiome
3.7. Association between Obesity, Microbiota Dysbiosis, and Neurodegenerative Pathogenesis
3.8. Use of Probiotic Therapy in Improving Women’s Health
4. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohajeri, M.H.; Brummer, R.J.M.; Rastall, R.A.; Weersma, R.K.; Harmsen, H.J.M.; Faas, M.; Eggersdorfer, M. The role of the microbiome for human health: From basic science to clinical applications. Eur. J. Nutr. 2018, 57, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Gregorczyk-Maślanka, K.; Kurzawa, R. Mikrobiota organizmu ludzkiego i jej wpływ na homeostazę immunologiczną—Część I Human microbiota. The impact on immune homeostasis—Part I. Alerg. Astma Immunol. 2016, 21, 146–150. [Google Scholar]
- Bartnicka, A.; Gałęcka, M.; Mazela, J. Wpływ czynników prenatalnych i postnatalnych na mikrobiotę jelitową noworodków. Stand. Med./Pediatr. 2016, 13, 165–172. [Google Scholar]
- Grot, M.; Krupa-Kotara, K.; Wypych-Ślusarska, A.; Grajek, M.; Białek-Dratwa, A. The Concept of Intrauterine Programming and the Development of the Neonatal Microbiome in the Prevention of SARS-CoV-2 Infection. Nutrients 2022, 14, 1702. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [Green Version]
- Nogal, A.; Valdes, A.M.; Menni, C. The role of short-chain fatty acids in the interplay between gut microbiota and diet in cardio-metabolic health. Gut Microbes 2021, 13, 1897212. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar Reddy, D. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef]
- Santhiravel, S.; Bekhit, A.E.-D.A.; Mendis, E.; Jacobs, J.L.; Dunshea, F.R.; Rajapakse, N.; Ponnampalam, E.N. The Impact of Plant Phytochemicals on the Gut Microbiota of Humans for a Balanced Life. Int. J. Mol. Sci. 2022, 23, 8124. [Google Scholar] [CrossRef]
- Winiarska-Mieczan, A.; Tomaszewska, E.; Donaldson, J.; Jachimowicz, K. The Role of Nutritional Factors in the Modulation of the Composition of the Gut Microbiota in People with Autoimmune Diabetes. Nutrients 2022, 14, 2498. [Google Scholar] [CrossRef]
- Altveş, S.; Altveş, S.; Yildiz, H.K.; Yildiz, H.K.; Vural, H.C.; Vural, H.C.; Altveş, S.; Altveş, S.; Yildiz, H.K.; Yildiz, H.K.; et al. Interaction of the microbiota with the human body in health and diseases. Biosci. Microbiota Food Health 2020, 39, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Morowitz, M.J.; Carlisle, E.M.; Alverdy, J.C. Contributions of Intestinal Bacteria to Nutrition and Metabolism in the Critically Ill. Surg. Clin. N. Am. 2011, 91, 771–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamczyk, K.; Garncarczyk, A.; Antończak, P. The mikrobiome of the skin. Przegl. Dermatol. 2018, 105, 285–297. [Google Scholar]
- Bloomfield, S.F.; Rook, G.A.; Scott, E.A.; Shanahan, F.; Stanwell-Smith, R.; Turner, P. Time to abandon hygiene hypo-thesis: New perspectives on allergic disease, the human microbiome, infectious disease prevention and the role of targeted hygiene. Perspect. Public Health 2016, 136, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, H. Acne, the Skin Microbiome, and Antibiotic Treatment. Am. J. Clin. Dermatol. 2019, 20, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Jańczewska, I.; Domżalska–Popadiuk, I. Znaczenie kolonizacji bakteryjnej przewodu pokarmowego noworodków donoszonych urodzonych drogą cięcia cesarskiego. Ann. Acad. Med. Gedan 2014, 44, 99–104. [Google Scholar]
- Rakowska, M.; Lichosik, M.; Kacik, J.; Kalicki, B. Wpływ mikrobioty na zdrowie człowieka. Pediatr. Med. Rodz 2016, 12, 404–412. [Google Scholar] [CrossRef]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef]
- Prendeville, S.; Sanders, C.; Sherry, J.; Costa, F. Circular Economy: Is It Enough. EcoDesign Centre, Wales. 2014. Available online: http://www.edcw.org/en/resources/circulareconomy-it-enough (accessed on 21 July 2014).
- Pathak, J.L.; Yan, Y.; Zhang, Q.; Wang, L.; Ge, L. The role of oral microbiome in respiratory health and diseases. Respir. Med. 2021, 185, 106475. [Google Scholar] [CrossRef]
- Baldwin, H.E.; Friedman, N.D.; Martin, A.; Eng, R.; Seité, S. The role of cutaneous microbiota harmony in maintaining a functionalskin barrier. J. Drugs Dermatol. 2017, 16, 12–18. [Google Scholar]
- Belizário, J.E.; Faintuch, J.; Garay-Malpartida, H.M. Gut Microbiome Dysbiosis and Immunometabolism: New Frontiers for Treatment of Metabolic Diseases. Mediat. Inflamm. 2018, 2018, 2037838. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; Godoy-Vitorino, F.; Knight, R.; Blaser, M.J. Role of the microbiome in human development. Gut 2019, 68, 1108–1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engstrand, L.; Graham, D.Y. Microbiome and Gastric Cancer. Dig. Dis. Sci. 2020, 65, 865–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Zhang, P.; Zhang, X. Probiotics Regulate Gut Microbiota: An Effective Method to Improve Immunity. Molecules 2021, 26, 6076. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Dittoe, D.K.; Pavilidis, H.O.; Chaney, W.E.; Yang, Y.; Ricke, S.C. Current Perspectives and Potential of Probiotics to Limit Foodborne Campylobacter in Poultry. Front. Microbiol. 2020, 11, 583429. [Google Scholar] [CrossRef]
- Jaworski, A.; Dudek, K.; Jurczak, I. Struktura i rola biologiczna mikrobioty przewodu pokarmowego człowieka w zdrowiu i w chorobie. JHSM 2016, 4, 37–61. [Google Scholar]
- Skonieczna-Żydecka, K.; Łoniewski, I.; Maciejewska, D.; Marlicz, W. Mikrobiota jelitowa i składniki pokarmowe jako determinanty funkcji układu nerwowego. Część I Mikrobiota przewodu pokarmowego. Aktualn. Neurol. 2017, 17, 181–188. [Google Scholar] [CrossRef]
- Juszczyk, K.; Grudlewska, K.; Mikucka, A.; Gospdarek-Komkowska, E. Przeszczepienie mikrobioty jelitowej—Metoda leczenia nawracających zakażeń o etiologii Clostridium difficile i innych chorób. Postępy Hig. Med. Dosw. 2017, 71, 220–226. [Google Scholar]
- Zhuang, L.; Chen, H.; Zhang, S.; Zhuang, J.; Li, Q.; Feng, Z. Intestinal Microbiota in Early Life and Its Implications on Childhood Health. Genom. Proteom. Bioinform. 2019, 17, 13–25. [Google Scholar] [CrossRef]
- Capuco, A.; Urits, I.; Hasoon, J.; Chun, R.; Gerald, B.; Wang, J.K.; Kassem, H.; Ngo, A.L.; Abd-Elsayed, A.; Simopoulos, T.; et al. Current Perspectives on Gut Microbiome Dysbiosis and Depression. Adv. Ther. 2020, 37, 1328–1346. [Google Scholar] [CrossRef] [Green Version]
- Al Bander, Z.; Nitert, M.D.; Mousa, A.; Naderpoor, N. The Gut Microbiota and Inflammation: An Overview. Int. J. Environ. Res. Public Health 2020, 17, 7618. [Google Scholar] [CrossRef]
- Benner, M.; Ferwerda, G.; Josten, I.; van der Molen, R.G. How uterine microbiota might be responsible for receptive, fer-tileendometrium. Hum. Reprod. Uptade 2018, 24, 393–415. [Google Scholar] [CrossRef] [Green Version]
- Franasiak, J.M.; Scott, R.T. Endometrial microbiome. Curr. Opin. Obstet. Gynecol. 2017, 29, 146–152. [Google Scholar] [CrossRef]
- Bogut, A.; Magryś, A. The road to success of coagulase-negative staphylococci: Clinical significance of small colony variants and their pathogenic role in persistent infections. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 2249–2270. [Google Scholar] [CrossRef]
- Becker, K.; Heilmann, C.; Peters, G. Coagulase-Negative Staphylococci. Clin. Microbiol. Rev. 2014, 27, 870–926. [Google Scholar] [CrossRef] [Green Version]
- Malinowska, M.; Tokarz-Deptuła, B.; Deptuła, W. Mikrobiom człowieka. Postępy Mikrobiol. 2017, 56, 33–42. [Google Scholar]
- Górecka, D.; Nowińska, A.; Augustynowicz–Kopeć, E. Mikrobiota układu oddechowego. Pneumonol. Alergol. Pol. 2014, 82, 481–485. [Google Scholar] [PubMed] [Green Version]
- Macura, B.; Majewska-Szczepanik, M.; Strzępa, A.; Szczepanik, M. Wpływ mikrobioty macicy na zdrowie kobiety i jej potomstwa. Med. Og. Nauk. Zdr. 2020, 26, 230–239. [Google Scholar] [CrossRef]
- Bomba-Opoń, D.; Drews, K.; Huras, H.; Laudański, P.; Paszkowski, T.; Wielgoś, M. Indukcja porodu—Algorytmy kliniczne. Wytyczne Polskiego Towarzystwa Ginekologicznego. GiPP 2018, 3, 23–29. [Google Scholar]
- Castanheira, C.P.; Sallas, M.L.; Nunes, R.A.L.; Lorenzi, N.P.C.; Termini, L. Microbiome and Cervical Cancer. Pathobiology 2020, 88, 187–197. [Google Scholar] [CrossRef]
- Lim, S.; Rajagopal, S.; Jeong, Y.R.; Nzegwu, D.; Wright, M.L. Group B Streptococcus and the vaginal microbiome among pregnant women: A systematic review. PeerJ 2021, 9, e11437. [Google Scholar] [CrossRef]
- Wu, M.; Guo, Y.; Wei, S.; Xue, L.; Tang, W.; Chen, D.; Xiong, J.; Huang, Y.; Fu, F.; Wu, C.; et al. Biomaterials and advanced technologies for the evaluation and treatment of ovarian aging. J. Nanobiotechnol. 2022, 20, 374. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.E. Endocrinology of the Menopause. Endocrinol. Metab. Clin. N. Am. 2015, 44, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Auriemma, R.S.; Scairati, R.; del Vecchio, G.; Liccardi, A.; Verde, N.; Pirchio, R.; Pivonello, R.; Ercolini, D.; Colao, A. The Vaginal Microbiome: A Long Urogenital Colonization Throughout Woman Life. Front. Cell. Infect. Microbiol. 2021, 11, 613. [Google Scholar] [CrossRef]
- Shen, L.; Zhang, W.; Yuan, Y.; Zhu, W.; Shang, A. Vaginal microecological characteristics of women in different physiological and pathological period. Front. Cell. Infect. Microbiol. 2022, 12, 1071. [Google Scholar] [CrossRef]
- Gupta, P.; Singh, M.P.; Goyal, K. Diversity of Vaginal Microbiome in Pregnancy: Deciphering the Obscurity. Front. Public Health 2020, 8, 326. [Google Scholar] [CrossRef]
- Song, S.D.; Acharya, K.D.; Zhu, J.E.; Deveney, C.M.; Walther-Antonio, M.R.S.; Tetel, M.J.; Chia, N. Daily Vaginal Microbiota Fluctuations Associated with Natural Hormonal Cycle, Contraceptives, Diet, and Exercise. Msphere 2020, 5. [Google Scholar] [CrossRef]
- Balle, C.; Konstantinus, I.N.; Jaumdally, S.Z.; Havyarimana, E.; Lennard, K.; Esra, R.; Barnabas, S.L.; Happel, A.-U.; Moodie, Z.; Gill, K.; et al. Hormonal contraception alters vaginal microbiota and cytokines in South African adolescents in a randomized trial. Nat. Commun. 2020, 11, 5578. [Google Scholar] [CrossRef]
- Chee, W.J.Y.; Chew, S.Y.; Than, L.T.L. Vaginal microbiota and the potential of Lactobacillus derivatives in maintaining vaginal health. Microb. Cell Factories 2020, 19, 203. [Google Scholar] [CrossRef]
- Geng, L.; Huang, W.; Jiang, S.; Zheng, Y.; Zhou, Y.; Zhou, Y.; Hu, J.; Li, P.; Tao, M. Effect of Menopausal Hormone Therapy on the Vaginal Microbiota and Genitourinary Syndrome of Menopause in Chinese Menopausal Women. Front. Microbiol. 2020, 11, 590877. [Google Scholar] [CrossRef]
- Qi, X.; Yun, C.; Pang, Y.; Qiao, J. The impact of the gut microbiota on the reproductive and metabolic endocrine system. Gut Microbes 2021, 13, 1894070. [Google Scholar] [CrossRef]
- Kwa, M.; Plottel, C.S.; Blaser, M.J.; Adams, S. The Intestinal Microbiome and Estrogen Receptor–Positive Female Breast Cancer. Gynecol. Oncol. 2016, 108, djw029. [Google Scholar] [CrossRef]
- Fuentes, N.; Silveyra, P. Estrogen receptor signaling mechanisms. Adv. Protein Chem. Struct. Biol. 2019, 116, 135–170. [Google Scholar] [CrossRef] [PubMed]
- Mulak, A. Sex hormones in the modulation of irritable bowel syndrome. World J. Gastroenterol. 2014, 20, 2433–2448. [Google Scholar] [CrossRef]
- Hussain, T.; Murtaza, G.; Kalhoro, D.H.; Kalhoro, M.S.; Metwally, E.; Chughtai, M.I.; Mazhar, M.U.; Khan, S.A. Relationship between gut microbiota and host-metabolism: Emphasis on hormones related to reproductive function. Anim. Nutr. 2021, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Coombes, Z.; Yadav, V.; McCoubrey, L.; Freire, C.; Basit, A.; Conlan, R.; Gonzalez, D. Progestogens Are Metabolized by the Gut Microbiota: Implications for Colonic Drug Delivery. Pharmaceutics 2020, 12, 760. [Google Scholar] [CrossRef] [PubMed]
- D’Afflitto, M.M.; Upadhyaya, A.; Green, A.M.; Peiris, M. Association Between Sex Hormone Levels and Gut Microbiota Composition and Diversity—A Systematic Review. J. Clin. Gastroenterol. 2022, 56, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-Z.; Sun, J.-H.; Wang, W.-J. Gut microbiota in gastrointestinal diseases during pregnancy. World J. Clin. Cases 2022, 10, 2976–2989. [Google Scholar] [CrossRef]
- Amir, M.; Brown, J.; Rager, S.; Sanidad, K.; Ananthanarayanan, A.; Zeng, M. Maternal Microbiome and Infections in Pregnancy. Microorganisms 2020, 8, 1996. [Google Scholar] [CrossRef]
- Plows, J.F.; Stanley, J.L.; Baker, P.N.; Reynolds, C.M.; Vickers, M.H. The Pathophysiology of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2018, 19, 3342. [Google Scholar] [CrossRef]
- Hasain, Z.; Mokhtar, N.M.; Kamaruddin, N.A.; Ismail, N.A.M.; Razalli, N.H.; Gnanou, J.V.; Ali, R.A.R. Gut Microbiota and Gestational Diabetes Mellitus: A Review of Host-Gut Microbiota Interactions and Their Therapeutic Potential. Front. Cell. Infect. Microbiol. 2020, 10, 188. [Google Scholar] [CrossRef]
- Knezevic, J.; Starchl, C.; Berisha, A.T.; Amrein, K. Thyroid-Gut-Axis: How Does the Microbiota Influence Thyroid Function? Nutrients 2020, 12, 1769. [Google Scholar] [CrossRef] [PubMed]
- Bargiel, P.; Szczuko, M.; Stachowska, L.; Prowans, P.; Czapla, N.; Markowska, M.; Petriczko, J.; Kledzik, J.; Jędrzejczyk-Kledzik, A.; Palma, J.; et al. Microbiome Metabolites and Thyroid Dysfunction. J. Clin. Med. 2021, 10, 3609. [Google Scholar] [CrossRef] [PubMed]
- Paray, B.; Albeshr, M.; Jan, A.; Rather, I. Leaky Gut and Autoimmunity: An Intricate Balance in Individuals Health and the Diseased State. Int. J. Mol. Sci. 2020, 21, 9770. [Google Scholar] [CrossRef] [PubMed]
- Madison, A.; Kiecolt-Glaser, J.K. Stress, depression, diet, and the gut microbiota: Human–bacteria interactions at the core of psychoneuroimmunology and nutrition. Curr. Opin. Behav. Sci. 2019, 28, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Lu, G.; Gao, D.; Lv, Z.; Li, D. The relationships between the gut microbiota and its metabolites with thyroid diseases. Front. Endocrinol. 2022, 13, 943408. [Google Scholar] [CrossRef]
- Wang, B.; Xu, Y.; Hou, X.; Li, J.; Cai, Y.; Hao, Y.; Ouyang, Q.; Wu, B.; Sun, Z.; Zhang, M.; et al. Small Intestinal Bacterial Overgrowth in Subclinical Hypothyroidism of Pregnant Women. Front. Endocrinol. 2021, 12, 604070. [Google Scholar] [CrossRef] [PubMed]
- Sawicka-Gutaj, N.; Gruszczyński, D.; Zawalna, N.; Nijakowski, K.; Muller, I.; Karpiński, T.; Salvi, M.; Ruchała, M. Microbiota Alterations in Patients with Autoimmune Thyroid Diseases: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 13450. [Google Scholar] [CrossRef]
- Thackray, V.G. Sex, Microbes, and Polycystic Ovary Syndrome. Trends Endocrinol. Metab. 2019, 30, 54–65. [Google Scholar] [CrossRef]
- Giampaolino, P.; Foreste, V.; Di Filippo, C.; Gallo, A.; Mercorio, A.; Serafino, P.; Improda, F.; Verrazzo, P.; Zara, G.; Buonfantino, C.; et al. Microbiome and PCOS: State-Of-Art and Future Aspects. Int. J. Mol. Sci. 2021, 22, 2048. [Google Scholar] [CrossRef] [PubMed]
- Verdugo-Meza, A.; Ye, J.; Dadlani, H.; Ghosh, S.; Gibson, D.L. Connecting the Dots Between Inflammatory Bowel Disease and Metabolic Syndrome: A Focus on Gut-Derived Metabolites. Nutrients 2020, 12, 1434. [Google Scholar] [CrossRef]
- Rizzetto, L.; Fava, F.; Tuohy, K.M.; Selmi, C. Connecting the immune system, systemic chronic inflammation and the gut microbiome: The role of sex. J. Autoimmun. 2018, 92, 12–34. [Google Scholar] [CrossRef] [PubMed]
- Mihajlovic, J.; Leutner, M.; Hausmann, B.; Kohl, G.; Schwarz, J.; Röver, H.; Stimakovits, N.; Wolf, P.; Maruszczak, K.; Bastian, M.; et al. Combined hormonal contraceptives are associated with minor changes in composition and diversity in gut microbiota of healthy women. Environ. Microbiol. 2021, 23, 3037–3047. [Google Scholar] [CrossRef] [PubMed]
- Acharya, K.D.; Gao, X.; Bless, E.P.; Chen, J.; Tetel, M.J. Estradiol and high fat diet associate with changes in gut microbiota in female ob/ob mice. Sci. Rep. 2019, 9, 20192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marizzoni, M.; Provasi, S.; Cattaneo, A.; Frisoni, G.B. Microbiota and neurodegenerative diseases. Curr. Opin. Neurol. 2017, 30, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.R.; Banerjee, S. Gut microbiota in neurodegenerative disorders. J. Neuroimmunol. 2019, 328, 98–104. [Google Scholar] [CrossRef]
- Ghaisas, S.; Maher, J.; Kanthasamy, A. Gut microbiome in health and disease: Linking the microbiome–gut–brain axis and environmental factors in the pathogenesis of systemic and neurodegenerative diseases. Pharmacol. Ther. 2016, 158, 52–62. [Google Scholar] [CrossRef] [Green Version]
- Cenit, M.C.; Sanz, Y.; Codoñer-Franch, P. Influence of gut microbiota on neuropsychiatric disorders. World J. Gastroenterol. 2017, 23, 5486–5498. [Google Scholar] [CrossRef] [Green Version]
- Chandra, S.; Alam, T.; Dey, J.; Sasidharan, B.C.P.; Ray, U.; Srivastava, A.K.; Gandhi, S.; Tripathi, P.P. Healthy Gut, Healthy Brain: The Gut Microbiome in Neurodegenerative Disorders. Curr. Top. Med. Chem. 2020, 20, 1142–1153. [Google Scholar] [CrossRef]
- Luan, H.; Wang, X.; Cai, Z. Mass spectrometry-based metabolomics: Targeting the crosstalk between gut microbiota and brain in neurodegenerative disorders. Mass Spectrom. Rev. 2017, 38, 22–33. [Google Scholar] [CrossRef]
- De JR De-Paula, V.; Forlenza, A.S.; Forlenza, O.V. Relevance of gutmicrobiota in cognition, behaviour and Alzheimer’s disease. Pharmacol. Res. 2018, 136, 29–34. [Google Scholar] [CrossRef]
- Szewczyk, A.; Witecka, A.; Kiersztan, A. Rola mikrobioty jelitowej w patogenezie chorób neuropsychiatrycznych i neurodegeneracyjnych. Postępy Hig. Med. Dosw. 2019, 73, 865–886. [Google Scholar] [CrossRef]
- Hasegawa, S.; Goto, S.; Tsuji, H.; Okuno, T.; Asahara, T.; Nomoto, K.; Shibata, A.; Fujisawa, Y.; Minato, T.; Okamoto, A.; et al. Intestinal Dysbiosis and Lowered Serum Lipopolysaccharide-Binding Protein in Parkinson’s Disease. PLoS ONE 2015, 10, e0142164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fasano, A.; Bove, F.; Gabrielli, M.; Petracca, M.; Zocco, M.A.; Ragazzoni, E.; Barbaro, F.; Piano, C.; Fortuna, S.; Tortora, A.; et al. The role of small intestinal bacterial overgrowth in Parkinson’s disease. Mov. Disord. 2013, 28, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Pradhan, L.K.; Sahoo, P.K.; Jena, K.K.; Chauhan, N.R.; Chauhan, S.; Das, S.K. Unravelling the potential of gut microbiota in sustaining brain health and their current prospective towards development of neurotherapeutics. Arch. Microbiol. 2021, 203, 2895–2910. [Google Scholar] [CrossRef] [PubMed]
- Spielman, L.J.; Gibson, D.L.; Klegeris, A. Unhealthy gut, unhealthy brain: The role of the intestinal microbiota in neurodegenerative diseases. Neurochem. Int. 2018, 120, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Chidambaram, S.B.; Essa, M.M.; Rathipriya, A.; Bishir, M.; Ray, B.; Mahalakshmi, A.M.; Tousif, A.; Sakharkar, M.K.; Kashyap, R.S.; Friedland, R.P.; et al. Gut dysbiosis, defective autophagy and altered immune responses in neurodegenerative diseases: Tales of a vicious cycle. Pharmacol. Ther. 2021, 231, 107988. [Google Scholar] [CrossRef] [PubMed]
- Grajek, M.; Krupa-Kotara, K.; Białek-Dratwa, A.; Sobczyk, K.; Grot, M.; Kowalski, O.; Staśkiewicz, W. Nutrition and mental health: A review of current knowledge about the impact of diet on mental health. Front. Nutr. 2022, 9. [Google Scholar] [CrossRef] [PubMed]
- Vamanu, E.; Rai, S. The Link between Obesity, Microbiota Dysbiosis, and Neurodegenerative Pathogenesis. Diseases 2021, 9, 45. [Google Scholar] [CrossRef]
- Banach, K.; Glibowski, P. Wpływ modyfikacji składu mikrobioty jelitowej na zmianę parametrów antropome-trycznych u osób z nadmierną masą ciała. Postepy Hig. Med. Dosw. 2018, 72, 913–923. [Google Scholar] [CrossRef]
- Cuevas-Sierra, A.; Ramos-Lopez, O.; Riezu-Boj, J.; Milagro, F.; Martinez, J. Diet, Gut Microbiota, and Obesity: Links with Host Genetics and Epigenetics and Potential Applications. Adv. Nutr. 2019, 10, S17–S30. [Google Scholar] [CrossRef] [Green Version]
- Kadooka, Y.; Sato, M.; Imaizumi, K.; Ogawa, A.; Ikuyama, K.; Akai, Y.; Okano, M.; Kagoshima, M.; Tsuchida, T. Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur. J. Clin. Nutr. 2010, 64, 636–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadooka, Y.; Sato, M.; Ogawa, A.; Miyoshi, M.; Uenishi, H.; Ogawa, H.; Ikuyama, K.; Kagoshima, M.; Tsuchida, T. Effect of Lactobacillus gasseri SBT2055 in fermented milk on abdominal adiposity in adults in a randomised controlled trial. Br. J. Nutr. 2013, 110, 1696–1703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavallari, J.F.; Fullerton, M.D.; Duggan, B.M.; Foley, K.P.; Denou, E.; Smith, B.K.; Desjardins, E.M.; Henriksbo, B.D.; Kim, K.J.; Tuinema, B.R.; et al. Muramyl Dipeptide-Based Postbiotics Mitigate Obesity-Induced Insulin Resistance via IRF4. Cell Metab. 2017, 25, 1063–1074.e3. [Google Scholar] [CrossRef]
- Dewulf, E.M.; Cani, P.; Claus, S.; Fuentes, S.; Puylaert, P.G.B.; Neyrinck, A.; Bindels, L.B.; De Vos, W.M.; Gibson, G.R.; Thissen, J.-P.; et al. Insight into the prebiotic concept: Lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women. Gut 2012, 62, 1112–1121. [Google Scholar] [CrossRef]
- Vrieze, A.; Van Nood, E.; Holleman, F.; Salojärvi, J.; Kootte, R.S.; Bartelsman, J.F.; Dallinga-Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of Intestinal Microbiota From Lean Donors Increases Insulin Sensitivity in Individuals With Metabolic Syndrome. Gastroenterology 2012, 143, 913–916.e7. [Google Scholar] [CrossRef] [PubMed]
- Palleja, A.; Kashani, A.; Allin, K.H.; Nielsen, T.; Zhang, C.; Li, Y.; Brach, T.; Liang, S.; Feng, Q.; Jørgensen, N.B.; et al. Roux-en-Y gastric bypass surgery of morbidly obese patients induces swift and persistent changes of the individual gut microbiota. Genome Med. 2016, 8, 67. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; DiBaise, J.K.; Zuccolo, A.; Kudrna, D.; Braidotti, M.; Yu, Y.; Parameswaran, P.; Crowell, M.D.; Wing, R.; Rittmann, B.E.; et al. Human Gut Microbiota in Obesity and after Gastric Bypass. Proc. Natl. Acad. Sci. USA 2009, 106, 2365–2370. [Google Scholar] [CrossRef] [Green Version]
- Nicoletti, C.F.; Cortes-Oliveira, C.; Pinhel, M.A.S.; Nonino, C.B. Bariatric Surgery and Precision Nutrition. Nutrients 2017, 9, 974. [Google Scholar] [CrossRef]
- Takahashi, T.; Fukudome, H.; Ueno, H.M.; Watanabe-Matsuhashi, S.; Nakano, T.; Kobayashi, T.; Ishimaru, K.; Nakao, A. Effects of Probiotic Supplementation on TGF-β1, TGF-β2, and IgA Levels in the Milk of Japanese Women: An Open-Label Pilot Study. Front. Nutr. 2019, 6, 128. [Google Scholar] [CrossRef]
- Qiu, G.; Yu, Y.; Wang, Y.; Wang, X. The significance of probiotics in preventing radiotherapy-induced diarrhea in patients with cervical cancer: A systematic review and meta-analysis. Int. J. Surg. 2019, 65, 61–69. [Google Scholar] [CrossRef]
- Shafie, M.; Rad, A.H.; Mohammad-Alizadeh-Charandabi, S.; Mirghafourvand, M. The effect of probiotics on mood and sleep quality in postmenopausal women: A triple-blind randomized controlled trial. Clin. Nutr. ESPEN 2022, 50, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Husain, S.; Allotey, J.; Drymoussi, Z.; Wilks, M.; Fernandez-Felix, B.M.; Whiley, A.; Dodds, J.; Thangaratinam, S.; McCourt, C.; Prosdocimi, E.M.; et al. Effects of oral probiotic supplements on vaginal microbiota during pregnancy: A randomised, double-blind, placebo-controlled trial with microbiome analysis. BJOG Int. J. Obstet. Gynaecol. 2019, 127, 275–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van De Wijgert, J.; Verwijs, M.C. Lactobacilli-containing vaginal probiotics to cure or prevent bacterial or fungal vaginal dysbiosis: A systematic review and recommendations for future trial designs. BJOG Int. J. Obstet. Gynaecol. 2019, 127, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Feng, Q.; Zheng, S.; Xiao, X. The effects of probiotics supplementation on metabolic health in pregnant women: An evidence based meta-analysis. PLoS ONE 2018, 13, e0197771. [Google Scholar] [CrossRef] [Green Version]
- Martoni, C.J.; Frederiksen, A.K.S.; Damholt, A.; Leyer, G. Effects of a 10-Strain Oral Probiotic on Parameters of Vaginal Health and Microbial Community: A Pilot Clinical Study. Int. J. Women’s Health 2022, 14, 29–39. [Google Scholar] [CrossRef]
- Steenbergen, L.; Sellaro, R.; van Hemert, S.; Bosch, J.A.; Colzato, L.S. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav. Immun. 2015, 48, 258–264. [Google Scholar] [CrossRef] [Green Version]
- Behrouzi, A.; Nafari, A.H.; Siadat, S.D. The significance of microbiome in personalized medicine. Clin. Transl. Med. 2019, 8, 16. [Google Scholar] [CrossRef]
- Cani, P.D.; De Vos, W.M. Next-Generation Beneficial Microbes: The Case of Akkermansia muciniphila. Front. Microbiol. 2017, 8, 1765. [Google Scholar] [CrossRef]

| Source | Sample | Probiotic Ingredient or Other Intervention | Effect of Therapy |
|---|---|---|---|
| Takahashi et al. [100] | An open-label pilot trial evaluating the safety of probiotic supplementation in lactating women with a 2-month history of allergies. | L. casei, B. longum, B. coagulans | Probiotic supplementation may affect TGF-β levels in human milk while finding a positive effect of probiotic supplementation requires further research. |
| Qiu et al. [101] | Systematic review and meta-analysis were conducted to evaluate the efficacy and safety of probiotics in the prevention of radiotherapy-induced diarrhea in patients with cervical cancer. | Mainly bacteria of the Lactobacillus and Bifidobacterium species | Probiotic supplementation may reduce the incidence of radiotherapy-induced diarrhea in cervical cancer patients. |
| Shafie et al. [102] | A triple-blind randomized controlled trial was conducted on 66 postmenopausal women aged 45–55 years. | B. lactis, L. acidophilus | There were improvements in anxiety, stress, and quality of life in postmenopausal women. |
| Husain et al. [103] | Randomized, double-blind, placebo-controlled trial conducted among women aged 16 years or older recruited at 9–14 weeks gestation. | L. rhamnosus GR-1, L. reuteri RC-14 | Probiotics taken orally from early pregnancy did not modify the vaginal microbiota. |
| van de Wijgert et al. [104] | A systematic review evaluating the effect of vaginal probiotics on the cure and/or recurrence of bacterial vaginosis and vulvovaginal candidiasis. | Lactobacillus strains | Probiotics are promising for the treatment and prevention of bacterial vaginosis, but much less so for the treatment and prevention of vulvovaginal candidiasis. |
| Zheng et al. [105] | Review article evaluating the effects of probiotics supplementation on metabolic health and pregnancy complications in pregnant women. | Mainly bacteria of the Lactobacillus and Bifidobacterium species | Probiotic supplementation during pregnancy has beneficial effects on glucose metabolism but not lipid metabolism among pregnant women. |
| Martoni et al. [106] | A pilot clinical study investigating the clinical effects of a 10-strain probiotic on parameters of vaginal health in women with intermediate Nugent score or vaginal pH > 4.5. | L. acidophilus DDS-1, L. gasseri UALg-05, L. plantarum UALp-05, L. rhamnosus UALr-06, L. reuteri UALre-16, L. paracasei UALpc-04, L. crispatus UALcr-35, L. brevis UALbr-02, B. longum subsp. longum UABl-14, B. animalis subsp. lactis UABla-12 | The probiotic product tested helped to significantly lower vaginal pH in women with intermediate Nugent score or elevated vaginal pH. |
| Sarkar et al. [76] | Review on the role of microbiota and probiotics in neurodegenerative diseases. | Lactobacillus casei shirota, Bacillus spp. | Regular consumption of a probiotic beverage containing Lactobacillus casei shirota has a positive effect on the gut microbiota in patients with Parkinson’s disease, while Bacillus spp. may have a positive effect on dopamine synthesis. |
| Cenit et al. [78] | Review the role of the gut microbiota in brain development and function. | Lactobacillus rhamnosus, Lactobacillus helveticus, Bifidobacterium infantis, Bifidobacterium longum, Bifidobacterium breve | Probiotic therapies using the aforementioned strains had an effect on relieving depressive symptoms. |
| Luan et al. [80] | Review of recent metabolomic research findings on the metabolic pathways that exist between the gut microbiota and the brain. | Lactobacillus and Bifidobacterium species | Lactobacillus and Bifidobacterium can produce gamma-aminobutyric acid (GABA), which positively affects the exchange of signals between neurons. |
| Banerjee et al. [85] | Review article evaluating the role of gut microbiota in pathogenesis of various neurological conditions. | Bifidobacterium infantis, Bifidobacterium spp., Bacillus spp., Lactobacillus spp., Streptococcus, and Enterococcus spp. | Bifidobacterium infantis increases plasma tryptophan, which upregulates serotonin; Bifidobacterium spp. synthesise GABA, Bacillus spp. synthesize norepinephrine and dopamine, Lactobacillus spp. synthesize acetylcholine, Streptococcus, and Enterococcus spp. produce serotonin. Probiotic therapy could therefore affect mood and cognitive function. |
| Steenbergen et al. [107] | A triple-blind, placebo-controlled study of 20 healthy participants without current mood disorders who received a 4-week intervention with multispecies probiotic foods and 20 control participants receiving a placebo. | Bifidobacterium bifidum W23, Bifidobacterium lactis W52, Lactobacillus acidophilus W37, Lactobacillus brevis W63, Lactobacillus casei W56, Lactobacillus salivarius W24 and Lactococcus lactis (W19 and W58) | Probiotic therapy improved mood in depressed patients and reduced negative thoughts. |
| Grajek et al. [88] | Review article on the impact of lifestyle and nutrition on mental health. | Lactobacillus helveticus, Bifidobacterium longum | The additional use of psychobiotics may prove effective in the treatment of anxiety or depressive disorders. |
| Kadooka et al. [88] | Multicenter, double-blind, randomized, placebo-controlled intervention trial on 87 subjects with higher body mass index and abdominal visceral fat area. | Lactobacillus gasseri SBT2055 | After 12 weeks, a reduction in body weight, visceral and subcutaneous fat, BMI, waist and hip circumference, and an increase in serum adiponectin levels were observed. |
| Dewulf et al. [95] | A double-blind, placebo-controlled, intervention study that used prebiotic supplementation with inulin-type fructans (ITFs) in women with obesity. | Inulin/oligofructose 50/50 mix (prebiotic) | The use of ITF prebiotics also reduced the abundance of Bacteroides intestinalis, Bacteroides vulgatus, and Propionibacterium, which was associated with a slight decrease in fat mass. It has been shown that the implementation of ITF prebiotics can help delay or prevent obesity-related comorbidities. |
| Vamanu et al. [89] | Review article on the alleviation of human dysbiosis in degenerative diseases and obesity. | Lactobacillus curvatus HY7601, Lactobacillus plantarum KY1032; Lactobacillus reuteri | The therapy has resulted in the regulation of pro-inflammatory genes in adipose tissue and fatty acid oxidation genes in the liver. Lactobacillus reuteri has anti-inflammatory effects due to its role in controlling interleukin (IL)-10 cytokine synthesis. |
| Non-invasive Biomarkers to Help Diagnose and Stage of Disease |
|---|
| identification of women at risk |
| determination of the disease phenotype |
| Treatment |
| Intestinal barrier integrity (signaling for toll-like receptors, TLRs) |
| Modulation of intestinal dysbiosis |
| Antimicrobial and antifungal agents |
| prebiotics |
| probiotics |
| synbiotics |
| fecal microbiota transplantation (FMT) |
| bacteriophage therapy |
| Effects on the metabolism of the intestinal microbiota |
| postbiotics |
| molecule inhibition |
| genetically modified microbes |
| Personalized diet therapy |
| Pharmacomicrobiomics |
| appropriate selection of pharmaceuticals |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krupa-Kotara, K.; Helisz, P.; Gwioździk, W.; Grajek, M. The Importance of the Microbiota in Shaping Women’s Health—The Current State of Knowledge. Appl. Microbiol. 2023, 3, 11-34. https://doi.org/10.3390/applmicrobiol3010002
Krupa-Kotara K, Helisz P, Gwioździk W, Grajek M. The Importance of the Microbiota in Shaping Women’s Health—The Current State of Knowledge. Applied Microbiology. 2023; 3(1):11-34. https://doi.org/10.3390/applmicrobiol3010002
Chicago/Turabian StyleKrupa-Kotara, Karolina, Paulina Helisz, Weronika Gwioździk, and Mateusz Grajek. 2023. "The Importance of the Microbiota in Shaping Women’s Health—The Current State of Knowledge" Applied Microbiology 3, no. 1: 11-34. https://doi.org/10.3390/applmicrobiol3010002