Use of Overground Supported-Stepping Devices for Non-Ambulant Children, Adolescents, and Adults with Cerebral Palsy: A Scoping Review
Abstract
:1. Introduction
- What BSF, activity, and participation outcomes from supported-stepping device use have been reported?;
- Are there any data available regarding: rates of use; costs; abandonment; adverse effects; contraindications; or equipment type in relation to age, desired outcomes or GMFCS levels?;
- Do experiences of stakeholders differ in regard to supported-stepping device use?;
- What are the barriers and facilitators of supported-stepping device use?
2. Methods
2.1. Inclusion Criteria
2.2. Exclusion Criteria
- Studies including mainly individuals classified as GMFCS I–III or those able to walk independently or use hand-held walkers;
- Studies using robotic devices, exoskeletons or mechanical stepping devices with external power sources, stationary systems (confined to parallel bars or treadmill), ceiling suspension systems or institutional-type gait trainers (e.g., LiteGait) too large to be used in a home or classroom environment;
- Where overground gait or ambulatory training was the comparison intervention, studies were included only if a supported-stepping device was the main comparison and not used in combination with partial body-weight supported treadmill training or other powered intervention(s).
2.3. Data Extraction and Appraisal
3. Results
3.1. Syntheses and Guidelines
3.2. Primary Source Data
3.2.1. Sources of Evidence According to Supported-Stepping Device (SSD) Type
3.2.2. Use and Introduction of Supported-Stepping Devices (SSD) According to Age and GMFCS
3.2.3. Contraindications and Benefits of Supported-Stepping Device (SSD) Use
3.3. Expert Opinion
3.4. BSF, Activity and Participation Outcomes
3.5. Lived Experience of Supported-Stepping Device (SSD) Use
4. Discussion
4.1. Outcomes and Use of Supported-Stepping Devices (SSD)
4.1.1. Use of Supported-Stepping Devices (SSD) for BSF, Activity, and Participation Outcomes
4.1.2. Use of Supported-Stepping Devices (SSD) According to GMFCS Level
4.1.3. Use of Supported-Stepping Devices (SSD) in Relation to Equipment Type and Orientation
4.1.4. Stakeholder Experiences of Supported-Stepping Device (SSD) Use
4.1.5. Barriers and Facilitators of Supported-Stepping Device (SSD) Use
4.2. Recommendations for Clinical Practice
4.3. Recommendations for Future Research
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- MacLennan, A.H.; Lewis, S.; Moreno-De-Luca, A.; Fahey, M.; Leventer, R.J.; McIntyre, S.; Ben-Pazi, H.; Corbett, M.; Wang, X.; Baynam, G.; et al. Genetic or Other Causation Should Not Change the Clinical Diagnosis of Cerebral Palsy. J. Child Neurol. 2019, 34, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Smithers-Sheedy, H.; Badawi, N.; Blair, E.; Cans, C.; Himmelmann, K.; Krägeloh-Mann, I.; McIntyre, S.; Slee, J.; Uldall, P.; Watson, L.; et al. What constitutes cerebral palsy in the twenty-first century? Dev. Med. Child Neurol. 2014, 56, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Palisano, R.; Rosenbaum, P.; Walter, S.; Russell, D.; Wood, E.; Galuppi, B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev. Med. Child Neurol. 1997, 39, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Palisano, R.J.; Rosenbaum, P.; Bartlett, D.; Livingston, M.H. Content validity of the expanded and revised Gross Motor Function Classification System. Dev. Med. Child Neurol. 2008, 50, 744–750. [Google Scholar] [CrossRef]
- Bartlett, D.J.; Chiarello, L.A.; Mccoy, S.W.; Palisano, R.J.; Jeffries, L.; Fiss, A.L.; Rosenbaum, P.; Wilk, P. Determinants of gross motor function of young children with cerebral palsy: A prospective cohort study. Dev. Med. Child Neurol. 2014, 56, 275–282. [Google Scholar] [CrossRef]
- Rodby-Bousquet, E.; Czuba, T.; Hägglund, G.; Westbom, L. Postural asymmetries in young adults with cerebral palsy. Dev. Med. Child Neurol. 2013, 55, 1009–1015. [Google Scholar] [CrossRef] [Green Version]
- Casey, J.; Agustsson, A.; Rosenblad, A.; Rodby-Bousquet, E. Relationship between scoliosis, windswept hips and contractures with pain and asymmetries in sitting and supine in 2450 children with cerebral palsy. Disabil. Rehabil. 2022, 44, 6738–6743. [Google Scholar] [CrossRef]
- Casey, J.; Rosenblad, A.; Rodby-Bousquet, E. Postural asymmetries, pain, and ability to change position of children with cerebral palsy in sitting and supine: A cross-sectional study. Disabil. Rehabil. 2022, 44, 2363–2371. [Google Scholar] [CrossRef]
- Ganz, F.; Hammam, N.; Pritchard, L. Sedentary behavior and children with physical disabilities: A scoping review. Disabil. Rehabil. 2021, 43, 2963–2975. [Google Scholar] [CrossRef]
- Bailes, A.F.P.; Greve, K.P.; Long, J.; Kurowski, B.G.M.; Vargus-Adams, J.M.; Aronow, B.; Mitelpunkt, A. Describing the Delivery of Evidence-Based Physical Therapy Intervention to Individuals with Cerebral Palsy. Pediatr. Phys. Ther. 2021, 33, 65–72. [Google Scholar] [CrossRef]
- Maitre, N.L.; Chorna, O.; Romeo, D.M.; Guzzetta, A. A High-Risk Infant Follow-Up Program. Pediatr. Neurol. 2016, 65, 31–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Einspieler, C.; Bos, A.F.; Krieber-Tomantschger, M.; Alvarado, E.; Barbosa, V.M.; Bertoncelli, N.; Burger, M.; Chorna, O.; Del Secco, S.; DeRegnier, R.-A.; et al. Cerebral Palsy: Early Markers of Clinical Phenotype and Functional Outcome. J. Clin. Med. 2019, 8, 1616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lobo, M.A.; Harbourne, R.T.; Dusing, S.C.; McCoy, S.W. Grounding Early Intervention: Physical Therapy Cannot Just Be About Motor Skills Anymore. Phys. Ther. 2013, 93, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Roquet, M.; Garlantezec, R.; Remy-Neris, O.; Sacaze, E.; Gallien, P.; Ropars, J.; Houx, L.; Pons, C.; Brochard, S. From childhood to adulthood: Health care use in individuals with cerebral palsy. Dev. Med. Child Neurol. 2018, 60, 1271–1277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paleg, G.; Livingstone, R. Outcomes of gait trainer use in home and school settings for children with motor impairments: A systematic review. Clin. Rehabil. 2015, 29, 1077–1091. [Google Scholar] [CrossRef]
- World Health Organization. International Classification of Functioning, Disability & Health (ICF); World Health Organization: Geneva, Switzerland, 2001. [Google Scholar]
- Eisenberg, S.; Zuk, L.; Carmeli, E.; Katz-Leurer, M. Contribution of Stepping While Standing to Function and Secondary Conditions Among Children with Cerebral Palsy. Pediatr. Phys. Ther. 2009, 21, 79–85. [Google Scholar] [CrossRef]
- Wright, F.V.; Belbin, G.; Slack, M.; Jutai, J. An evaluation of the David Hart Walker Orthosis: A new assistive device for children with Cerebral Palsy. Physiother. Can. 1999, 51, 280–291. [Google Scholar]
- Paleg, G.; Livingstone, R. Evidence-informed clinical perspectives on selecting gait trainer features for children with cerebral palsy. Int. J. Ther. Rehabil. 2016, 23, 444–454. [Google Scholar] [CrossRef]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef] [Green Version]
- Levac, D.; Colquhoun, H.; O’Brien, K.K.; Levac, D.; Colquhoun, H.; O’Brien, K.K. Scoping studies: Advancing the methodology. Implement. Sci. 2010, 5, 69. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, K.K.; Colquhoun, H.; Levac, D.; Baxter, L.; Tricco, A.C.; Straus, S.; Wickerson, L.; Nayar, A.; Moher, D.; O’Malley, L. Advancing scoping study methodology: A web-based survey and consultation of perceptions on terminology, definition and methodological steps. BMC Health Serv. Res. 2016, 16, 305. [Google Scholar] [CrossRef] [Green Version]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLean, L.J.; Paleg, G.S.; Livingstone, R.W. Supported-standing interventions for children and young adults with non-ambulant cerebral palsy: A scoping review. Dev. Med. Child Neurol. 2022; in press. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, R.; Paleg, G. Measuring Outcomes for Children with Cerebral Palsy Who Use Gait Trainers. Technologies 2016, 4, 22. [Google Scholar] [CrossRef]
- Hong, Q.N.; Pluye, P.; Fàbregues, S.; Bartlett, G.; Boardman, F.; Cargo, M.; Dagenais, P.; Gagnon, M.-P.; Griffiths, F.; Nicolau, B.; et al. Mixed Methods Appraisal Tool (MMAT), Version 2018. User guide. McGill. 2018, pp. 1–11. Available online: http://mixedmethodsappraisaltoolpublic.pbworks.com/w/file/fetch/127916259/MMAT_2018_criteria-manual_2018-08-01_ENG.pdf (accessed on 11 December 2022).
- Rosenbaum, P.; Gorter, J.W. The ‘F-words’ in childhood disability: I swear this is how we should think! Child Care Health Dev. 2012, 38, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Longo, E.; Monteiro, R.; Hidalgo-Robles, A.; Paleg, G.; Shrader, C.; De Campos, A.C. Intervention ingredients and F-words in early intervention for children with cerebral palsy functioning at GMFCS IV and V: A scoping review protocol. Front. Rehabil. Sci. 2023, 4, 1110552. [Google Scholar] [CrossRef]
- Becker, C.; Hoppstadter, W. Gehend Spielend Handeln. Bachelor’s Thesis, Ergotherapie Hogeschool Zuyd, Heerlen, The Netherlands, 2003. [Google Scholar]
- Gibbins, K.; Baltzopoulos, V. The Effects of the David Hart Orthosis on the Gait Development of Children with Cerebral Palsy. Master’s Thesis, University of Liverpool, Liverpool, UK, 1994. [Google Scholar]
- Hoyme, D. Body Weight Supported Treadmill Training Versus Gait Trainer in Young Children with Cerebral Palsy. Ph.D. Thesis, 2016. [Google Scholar]
- Hallemans, A.; Cuppers, R.; Truijen, S.; Truijen, S.; Ego, F.; Moens, M.; Wenmakers, D.; Caers, P.; Lebeer, J. Walking in the Hibbot, an Innovative Walking Aid Improves Gait Characteristics in Children with Cerebral Palsy: A Cross-Sectional Study. Preprint 2020. [Google Scholar] [CrossRef]
- Jung, T.; Kim, Y.; Kelly, L.E.; Abel, M.F. Biomechanical and perceived differences between overground and treadmill walking in children with cerebral palsy. Gait Posture 2016, 45, 1–6. [Google Scholar] [CrossRef]
- Paleg, G.; Wright, J. Schizencephaly: Children with a rare developmental disorder are treated with physical therapy. Adv. Phys. Ther. PT Assist. 1998, 35–36. [Google Scholar]
- Paleg, G. Different approach–Physical therapy for a child with CHARGE syndrome: A contrast in practice patterns. Adv. PT 2003, 45–46. [Google Scholar]
- Parent, A.; Letellier, G.; Lachapelle, J.; Marois, P.; Larochelle, J.; Mohebbi, A.; Ballaz, L. Arm-free overground walking with partial body weight support in children with cerebral palsy: A case study. Gait Posture 2022, 97, S139–S140. [Google Scholar] [CrossRef]
- van der Krogt, M.M.; Sloot, L.H.; Harlaar, J. Overground versus self-paced treadmill walking in a virtual environment in children with cerebral palsy. Gait Posture 2014, 40, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J. MOVE program enables children to develop functional ambulatory skills. PT Bull. 1996, 4–5. [Google Scholar]
- Akhter, N. Mother’s Perceptions towards Using Functional Mobility Aids for Their Children with Cerebral Palsy. Bachelor’s Thesis, University of Dakha, Bangladesh, South Asia, 2015. [Google Scholar]
- Kassim, N.; Pattnaik, M.; Mohanty, P.; Kavi, M. Comparison of Integrated Task Oriented Bodyweight Supported Overground Training with Body-Weight Supported Treadmill Training to Improve Functional Mobility in Children with Spastic Diplegic Cerebral Palsy—A Single Blinded Randomized Control Trial. Biomed. Pharmacol. J. 2022, 15, 651–662. [Google Scholar] [CrossRef]
- Laksana, P.; Setyanto, R.; Herdiman, L. Redesign paediatric walker for children with spastic cerebral palsy using TRIZ Method. J. Sist. Dan. Manaj. Ind. 2021, 5, 8–14. [Google Scholar] [CrossRef]
- Lestari, N.T.; Susmartini, S.; Herdiman, L. Redesign paediatric walker for children with spastic cerebral palsy using TRIZ Method. J. Phys. Conf. Ser. 2020, 1450. [Google Scholar] [CrossRef]
- O’Handley, R.D.; Dadakhodjaeva, K.; Radley, K.C.; Dart, E.H. Promoting independent ambulation: A case study of an elementary school student with developmental disabilities. Res. Dev. Disabil. 2016, 56, 153–159. [Google Scholar] [CrossRef]
- Pool, D.; Elliott, C.; Willis, C.; Thornton, A. The Experience of Locomotor Training From the Perspectives of Therapists and Parents of Children With Cerebral Palsy. Front. Rehabil. Sci. 2021, 2, 740426. [Google Scholar] [CrossRef]
- Pool, D.; Valentine, J.; Taylor, N.F.; Bear, N.; Elliott, C. Locomotor and robotic assistive gait training for children with cerebral palsy. Dev. Med. Child Neurol. 2021, 63, 328–335. [Google Scholar] [CrossRef]
- Snarski, K.E. Effects of stepping practice with postural support on gross motor abilities of a pre-ambulatory child with cerebral palsy: A case report. Physiother. Theory Pr. 2021, 37, 234–241. [Google Scholar] [CrossRef]
- Su, I.Y.; Chung, K.K.; Chow, D.H. Treadmill training with partial body weight support compared with conventional gait training for low-functioning children and adolescents with nonspastic cerebral palsy: A two-period crossover study. Prosthetics Orthot. Int. 2013, 37, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, L. Why We Love Gait Training. Mobility Management. 2016. Available online: https://mobilitymgmt.com/articles/2016/04/01/gait-training.aspx (accessed on 11 December 2022).
- Bradbury, J.-A. “Standing tall”: An Analysis of Parents’ Evaluations of a Walker for Children with Cerebral Palsy. Master’s Thesis, University of Toronto, Toronto, ON, Canada, 1997. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71, PMCID:PMC8005924. [Google Scholar] [CrossRef] [PubMed]
- Amacher, R.; Steiner, N. Der einsatz von stehgestellen bei kindern mit infantiler cerebralparese aus ergotherapeutischer sicht. Bachelor’s Thesis, Zurich University of Applied Sciences, Winterthur, Switzerland, 2010. Available online: https://digitalcollection.zhaw.ch/bitstream/11475/322/1/Amacher_Steiner.pdf (accessed on 11 December 2022).
- Booth, A.T.C.; Buizer, A.; Meyns, P.; Lansink, I.L.B.O.; Steenbrink, F.; van der Krogt, M. The efficacy of functional gait training in children and young adults with cerebral palsy: A systematic review and meta-analysis. Dev. Med. Child Neurol. 2018, 60, 866–883. [Google Scholar] [CrossRef] [PubMed]
- Novak, I.; Morgan, C.; Fahey, M.; Finch-Edmondson, M.; Galea, C.; Hines, A.; Langdon, K.; Mc Namara, M.; Paton, M.C.; Popat, H.; et al. State of the Evidence Traffic Lights 2019: Systematic Review of Interventions for Preventing and Treating Children with Cerebral Palsy. Curr. Neurol. Neurosci. Rep. 2020, 20, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paleg, G.; Livingstone, R. Evidence-informed clinical perspectives on postural management for hip health in children and adults with non-ambulant cerebral palsy. J. Pediatr. Rehabil. Med. 2022, 15, 39–48. [Google Scholar] [CrossRef]
- Gannotti, M.E.P.; Liquori, B.M.M.; Thorpe, D.E.P.; Fuchs, R.K.P. Designing Exercise to Improve Bone Health Among Individuals With Cerebral Palsy. Pediatr. Phys. Ther. 2021, 33, 50–56. [Google Scholar] [CrossRef]
- Jackman, M.; Sakzewski, L.; Morgan, C.; Boyd, R.N.; Brennan, S.E.; Langdon, K.; Toovey, R.A.M.; Greaves, S.; Thorley, M.; Novak, I. Interventions to improve physical function for children and young people with cerebral palsy: International clinical practice guideline. Dev. Med. Child Neurol. 2022, 64, 536–549. [Google Scholar] [CrossRef]
- de Campos, A.C.; Hidalgo Robles, A.; Longo, E.; Shrader, C.; Paleg, G. Scoping review of early interventions for young children classified as Gross Motor Function Classification System (GMFCS) IV and V. Dev. Med. Child Neurol. 2023; submitted. [Google Scholar]
- Willoughby, K.L.; Dodd, K.J.; Shields, N.; Foley, S. Efficacy of Partial Body Weight–Supported Treadmill Training Compared With Overground Walking Practice for Children With Cerebral Palsy: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2010, 91, 333–339. [Google Scholar] [CrossRef]
- van der Putten, A.; Vlaskamp, C.; Reynders, K.; Nakken, H. Children with profound intellectual and multiple disabilities: The effects of functional movement activities. Clin. Rehabil. 2005, 19, 613–620. [Google Scholar] [CrossRef]
- Kuenzle, C.; Brunner, R. The Effects of the Norsk Funktion-Walking Orthosis on the Walking Ability of Children With Cerebral Palsy and Severe Gait Impairment. JPO J. Prosthet. Orthot. 2009, 21, 138–144. [Google Scholar] [CrossRef]
- Wright, F.V.; Jutai, J.W. Evaluation of the longer-term use of the David Hart Walker Orthosis by children with cerebral palsy: A 3-year prospective evaluation. Disabil. Rehabil. Assist. Technol. 2006, 1, 155–166. [Google Scholar] [CrossRef]
- Smati, S.; Pouliot-Laforte, A.; Chevalier, M.; Lemay, M.; Ballaz, L. Effect of power training on locomotion capacities in children with cerebral palsy with GMFCS level III–IV. Disabil. Rehabil. 2022, 623. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, M.; Tierney, S. Perspectives of children with physical disabilities, parents and physiotherapists on use of walkers and their potential to increase physical activity. A qualitative exploration. J. Child Health Care 2022, 1–21. [Google Scholar] [CrossRef] [PubMed]
- McKeever, P.; Rossen, B.E.; Scott, H.; Robinson-Vincent, K.; Wright, V. The significance of uprightness: Parents’ reflections on children’s responses to a hands-free walker for children. Disabil. Soc. 2013, 28, 380–392. [Google Scholar] [CrossRef]
- Rodríguez-Costa, I.; De la Cruz-López, I.; Fernández-Zárate, I.; Maldonado-Bascón, S.; Lafuente-Arroyo, S.; Nunez-Nagy, S. Benefits of a Low-Cost Walking Device in Children with Cerebral Palsy: A Qualitative Study. Int. J. Environ. Res. Public Health 2021, 18, 2808. [Google Scholar] [CrossRef]
- Paananen, L. Ihastuttaako vai vihastuttaako ? kävelyn apuvälineeseen. Master’s Thesis, Jamk University of Applied Sciences, Jyväskylä, Finland, 2009. Available online: https://www.theseus.fi/bitstream/handle/10024/6662/Paananen_Laila.pdf?sequence=1&isAllowed=y (accessed on 11 December 2022).
- Barnes, S.B.; Whinnery, K.W. Effects of Functional Mobility Skills Training for Young Students with Physical Disabilities. Except. Child. 2002, 68, 313–324. [Google Scholar] [CrossRef]
- Lancioni, G.E.; Singh, N.N.; O’Reilly, M.F.; Campodonico, F.; Piazzolla, G.; Scalini, L.; Oliva, D. Impact of favorite stimuli automatically delivered on step responses of persons with multiple disabilities during their use of walker devices. Res. Dev. Disabil. 2005, 26, 71–76. [Google Scholar] [CrossRef]
- Lancioni, G.; Nirbhay, N.; O’Reilly, F.; Campodonico, F.; Oliva, O.; Vigo, C. Promoting walker-assisted step responses by an adolescent with multiple disabilities through automatically delivered stimulation. J. Vis. Impair. Blind. 2005, 99, 109–113. [Google Scholar] [CrossRef]
- Lancioni, G.E.; Singh, N.N.; O’Reilly, M.F.; Sigafoos, J.; Oliva, D.; Scalini, L.; Castagnaro, F.; Di Bari, M. Promoting foot–leg movements in children with multiple disabilities through the use of support devices and technology for regulating contingent stimulation. Cogn. Process. 2007, 8, 279–283. [Google Scholar] [CrossRef]
- Lancioni, G.E.; Singh, N.N.; O’Reilly, M.F.; Sigafoos, J.; Oliva, D.; Piazzolla, G.; Pidala, S.; Smaldone, A.; Manfredi, F. Automatically Delivered Stimulation for Walker-Assisted Step Responses: Measuring its Effects in Persons with Multiple Disabilities. J. Dev. Phys. Disabil. 2007, 19, 1–13. [Google Scholar] [CrossRef]
- Lancioni, G.E.; De Pace, C.; Singh, N.N.; O’Reilly, M.F.; Sigafoos, J.; Didden, R. Promoting Step Responses of Children with Multiple Disabilities through a Walker Device and Microswitches with Contingent Stimuli. Percept. Mot. Ski. 2008, 107, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Lancioni, G.E.; Singh, N.N.; O’Reilly, M.F.; Sigafoos, J.; Oliva, D.; Smaldone, A.; La Martire, M.L.; Stasolla, F.; Castagnaro, F.; Groeneweg, J. Promoting ambulation responses among children with multiple disabilities through walkers and microswitches with contingent stimuli. Res. Dev. Disabil. 2010, 31, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Lancioni, G.E.; Singh, N.N.; O’Reilly, M.F.; Sigafoos, J.; Oliva, D.; Campodonico, F.; Buono, S. Walker devices and microswitch technology to enhance assisted indoor ambulation by persons with multiple disabilities: Three single-case studies. Res. Dev. Disabil. 2013, 34, 2191–2199. [Google Scholar] [CrossRef] [PubMed]
- Broadbent, J.; Woollam, P.J.; Major, R.; Stallard, J. Technical note: A rear support walking frame for severely disabled children with cerebral palsy: Initial development. Prosthet. Orthot. Int. 2000, 24, 233–240. [Google Scholar] [CrossRef] [Green Version]
- Camallonga, M.O. Protocolo de valoración fisioterápica para el correcto uso del NF Walker en niños con PCI. Master’s Thesis, Universidad CEU Cardenal Herrera, Valencia, Spain, 2013. Available online: https://repositorioinstitucional.ceu.es/bitstream/10637/6026/4/Protocolo_Orti%20Camallonga_TFG_2013.pdf (accessed on 11 December 2022).
- Gandarias Mendieta, I. Control Postural En Fisioterapia Pediátrica: Bipedestación Activa Y Compresión Dinámica. Jorn. Científicas La Soc. Española Rehabil. Infant. 2012, 2, 28–32. [Google Scholar]
- Livingstone, R.W.; Paleg, G.S.; Field, D.A. Supported Standing and Stepping Device Use in Young Children with Cerebral Palsy, Gross Motor Function Classification System III, IV and V: A Descriptive Study. Assist. Technol. 2023; in press. [Google Scholar]
- Low, S. Effects of the MOVE (Mobility Opportunities Via Education) curriculum on range of motion, motor skills, and functional mobility of children with severe multiple disabilities: A pilot program. Pediatr. Phys. Ther. 2005, 17, 94–95. [Google Scholar]
- Low, S.; Westcott-McCoy, S.; Adams, J.; Beling, J. A comparison of two support walkers on the gait parameters of children with cerebral palsy. Physiotherapy 2011, 97, eS709. [Google Scholar] [CrossRef] [Green Version]
- Martín Gómez, M.; Laguna Mena, C.; Martín Maroto, M.; Arroyo Riaño, M. Evaluation of the NF-Walker. English translation of article originally published in: Jornadas Cientificas de la Sociedad Espanola de Rehabilitacion Infantil. 2012. Available online: https://cdn2.hubspot.net/hubfs/2252381/Documentation%20_%20How%20to/NF-Walker/NF-Walker%20an%20evaluation.pdf (accessed on 11 December 2022).
- Martín Gómez, M.; Laguna Mena, C.; Martín Maroto, M.; Echevarria Ulloa, M.; Dumitrescu, A.; Arroyo Riaño, M. Evaluation and follow-up of NF-Walker technical aid during the transition to adult life. Dev. Med. Child Neurol. 2021, 63 (Suppl. S2), 42. [Google Scholar] [CrossRef]
- Paleg, V.S. Teaching older children with cerebral palsy to sit, stand and walk. Dev. Med. Child Neurol. 1997, 39 (Suppl. 75), 10–11. [Google Scholar]
- Sharma, M.; Bajracharya, S. Low cost modified reverse walker to assist children with cerebral palsy. RGUHS J. Allied Health Sci. 2021, 1, 18–24. Available online: https://journalgrid.com/view/article/rjahs/37 (accessed on 11 December 2022).
- Wright-Ott, C. Mobility Matters—Imbedding Hands-Free Locomotion Experiences into the Preschool and Elementary Curricula for Students with Severe Speech and Motor Impairment: The Bridge School Experience. Available online: https://curriculum.bridgeschool.org/wp-content/uploads/sites/5/2018/06/mobility_matters.pdf (accessed on 27 January 2023).
- Gandarias Mendieta, I.; (Clinica Pixuflitos, Bilboa, Spain). Personal communication, 2023.
- George, C.; Levin, W.; Ryan, J.M. The use and perception of support walkers for children with disabilities: A United Kingdom survey. BMC Pediatr. 2020, 20, 528. [Google Scholar] [CrossRef] [PubMed]
- Low, S.A.; McCoy, S.W.; Beling, J.; Adams, J. Pediatric Physical Therapists’ Use of Support Walkers for Children With Disabilities: A nationwide survey. Pediatr. Phys. Ther. 2011, 23, 381–389. [Google Scholar] [CrossRef]
- Peredo, D.E.; Davis, B.E.; Norvell, D.C.; Kelly, P.C. Medical equipment use in children with disabilities: A descriptive survey. J. Pediatr. Rehabil. Med. 2010, 3, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Altizer, W.; Paleg, G. Everyday with Emmitt. Directions 2020, 3, 42–47. Available online: https://issuu.com/nrrts/docs/directions_202020v3-full_20mag (accessed on 11 December 2022).
- Benson, K.; Capone, K.; Duch, K.; Palmer-Casey, C. Mobility supports in educational curriculum for children and youth with cerebral palsy. In Cerebral Palsy; Miller, F., Bachrach, S.J., Lennon, N., O’Neil, M.E., Eds.; Springer Nature: Berlin, Germany, 2020; pp. 2903–2916. [Google Scholar] [CrossRef]
- Carnevale, D. PS7 2: The Functional Architecture of a Gait Trainer. In Proceedings of the International Seating Symposium, Nashville, TN, USA, 26–28 February 2015; pp. 221–222. [Google Scholar]
- Farrell, E.; Naber, E.; Geigle, P. Description of a multifaceted rehabilitation program including overground gait training for a child with cerebral palsy: A case report. Physiother. Theory Pr. 2010, 26, 56–61. [Google Scholar] [CrossRef]
- George, F.K. The importance of static and dynamic posture: How making static equipment dynamic may improve movement and function of children with neurological impairment—A retrospective service evaluation. Assoc. Paediatr. Chart Physiother. J. 2018, 9, 56–62. [Google Scholar]
- Jones, M.A. Wheeled mobility options and indications for children and youth with cerebral palsy. In Cerebral Palsy; Miller, F., Bachrach, S.J., Lennon, N., O’Neil, M.E., Eds.; Springer Nature: Berlin, Germany, 2020; pp. 2949–2961. [Google Scholar] [CrossRef]
- Low, S. Comparison of two support walkers on the gait and mobility of a child with cerebral palsy: A case report. Pediatr. Phys. Ther. 2004, 16, 60–61. [Google Scholar]
- Paleg, G. Mobility Opportunities Via Education: (The MOVE story). Tomorrow’s PT 1997, 10–11. [Google Scholar]
- Paleg, G. Holoprosencephaly: Rare developmental disorder presents clinically as cerebral palsy. Adv. PT 1998, 34–35. [Google Scholar]
- Paleg, G. When Needs are Special: Determining Mobility Requirements for a Child with Joubert Syndrome. Available online: https://rehabpub.com/mobility/when-needs-are-special/ (accessed on 11 December 2022).
- Pope, E.; Tally, M. The importance of supporting the equipment needs of pediatric clients with complex neuromotor disorders. Directions 2022, 2, 32–41. Available online: https://issuu.com/nrrts/docs/directions_2022v2_full_issuu/s/15304057 (accessed on 11 December 2022).
- Whinnery, K.; Barnes, S. Mobility Training Using the MOVE® Curriculum A Parent’s View. Teach. Except. Child. 2002, 34, 44–50. Available online: http://cec.metapress.com/index/MM83015262427641.pdf (accessed on 11 December 2022). [CrossRef]
- Gordon, A.; Magill, R.; Paleg, G. Motor learning: Application of principles to pediatric rehabilitation. In Physical Therapy for Children, 6th ed.; Campbell, S., Linden, R., Palisano, R., Eds.; Elsevier: St. Louis, MI, USA, 2023; pp. 91–111. [Google Scholar]
- Kannegießer-leitner, C. The NF-Walker in the Rehabilitation of Children with a Pronounced Movement Disorder. Made for Movement Product Studies. Available online: https://cdn2.hubspot.net/hubfs/2252381/Documentation%20_%20Know-How/NF-Walker/NF-Walker%20in%20the%20rehabilitation%20of%20children%20with%20a%20pronounced%20movement%20disorder.pdf (accessed on 11 December 2022).
- Paleg, G. The MOVE approach to disabilities: Lifting bodies and spirits. PT OT Today 1997, 5, 12–27. [Google Scholar]
- Schneiders, S. Does Early Application of Medical Aid Devices Make Sense? Based on the Example of the NF Walker. Made for Movement Product Studies. Available online: https://cdn2.hubspot.net/hubfs/2252381/Documentation%20_%20How%20to/NF-Walker/NF-Walker%20Does%20early%20application%20of%20medical%20aid%20devices%20make%20sense.pdf (accessed on 11 December 2022).
- Schwerin, A.; Francke, A.; Weiß, P. NF-walker and botulinum toxin therapy for children with spastic tetraparesis. Neuropediatrics 2005, 36, 111. [Google Scholar] [CrossRef]
- Thompson, G. Children with Severe Disabilities and the MOVE Curriculum: Foundations of a Task-Oriented Therapy Approach; East River Press: New York, NY, USA, 2005. [Google Scholar]
- Bolton, M.; Donohoe, M. Ambulatory assistive devices for children and youth with cerebral palsy. In Cerebral Palsy; Miller, F., Bachrach, S.J., Lennon, N., O’Neil, M.E., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 2963–2975. [Google Scholar] [CrossRef]
- Covert, S.Y. Promoting Pediatric Participation. Rehab. Manag. 2019, 32, 20–23. Available online: http://digitaledition.rehabpub.com/rehabpub/diged/20191112/index.html (accessed on 11 December 2022).
- Gandarias Mendieta, I. NF-Walker: Dynamic and Mobile Standing Orthosis. Made for Movement Product Studies. 2008. Available online: https://cdn2.hubspot.net/hubfs/2252381/Documentation%20_%20Know-How/NF-Walker/NF-Walker%20Dynamic%20and%20mobile%20standing%20orthosis.pdf (accessed on 11 December 2022).
- Marquez, G.M. Promoting Function and Independence in the Cerebral Palsy Population. Rehab. Manag. 2019, 32, 24–26. Available online: http://digitaledition.rehabpub.com/rehabpub/diged/20190708/index.html (accessed on 11 December 2022).
- Thunberg, G.; Livingstone, R.; Buchholz, M.; Field, D. Environmental Adaptations. In Early Detection and Early Intervention in Developmental Motor Disorders; Hadders-Algra, M., Ed.; Mac Keith Press: London, UK, 2021; pp. 228–241. [Google Scholar] [CrossRef]
- Botega, R.; Medola, F.O.; Santos, C.B.A.; Silva, A.T.; Iunes, D.H.; Purquerio, B.D.M. A new walking aid with axillary support for children with cerebral palsy: Electromyographic evaluation. Disabil. Rehabil. Assist. Technol. 2013, 8, 507–510. [Google Scholar] [CrossRef]
- Meadows, C.; Meyerink, J.; Farley, R.; Gilmour, A. The Arrow Walker. Physiotherapy 1992, 78, 679–680. [Google Scholar] [CrossRef]
- Stallard, J.; Major, R.E.; Farmer, S.E. The potential for ambulation by severely handicapped cerebral palsy patients. Prosthet. Orthot. Int. 1996, 20, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Paleg, G.; Huang, M.; Gabela, S.C.V.; Sprigle, S.; Livingstone, R. Comparison of the inertial properties and forces required to initiate movement for three gait trainers. Assist. Technol. 2016, 28, 137–143. [Google Scholar] [CrossRef]
- Sabet, A.; Feldner, H.; Tucker, J.; Logan, S.W.; Galloway, J.C. ON Time Mobility: Advocating for Mobility Equity. Pediatr. Phys. Ther. 2022, 34, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Willerslev-Olsen, M.; Lund, M.C.; Lorentzen, J.; Barber, L.; Kofoed-Hansen, M.; Nielsen, J.B. Impaired muscle growth precedes development of increased stiffness of the triceps surae musculotendinous unit in children with cerebral palsy. Dev. Med. Child Neurol. 2018, 60, 672–679. [Google Scholar] [CrossRef] [Green Version]
- Shin, Y.-K.; Yoon, Y.K.; Chung, K.B.; Rhee, Y.; Cho, S.-R. Patients with non-ambulatory cerebral palsy have higher sclerostin levels and lower bone mineral density than patients with ambulatory cerebral palsy. Bone 2017, 103, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Shortland, A. Muscle deficits in cerebral palsy and early loss of mobility: Can we learn something from our elders? Dev. Med. Child Neurol. 2009, 51, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Verschuren, O.; Peterson, M.D.; Balemans, A.C.; Hurvitz, E.A. Exercise and physical activity recommendations for people with cerebral palsy. Dev. Med. Child Neurol. 2016, 58, 798–808. [Google Scholar] [CrossRef]
- Verschuren, O.; Hulst, R.Y.; Voorman, J.; Pillen, S.; Luitwieler, N.; Dudink, J.; Gorter, J.W. 24-hour activity for children with cerebral palsy: A clinical practice guide. Dev. Med. Child Neurol. 2020, 63, 54–59. [Google Scholar] [CrossRef]
- Tedla, J.S.; Ganesan, S.; Katragadda, S. Inter-rater reliability of the Top Down Motor Milestone Test: A cross-sectional study. Clin. Rehabil. 2009, 23, 725–729. [Google Scholar] [CrossRef]
- Bradbury, M.; Bennison, E.; Mason, H.; Gregory, J. Tools for participation: Living aids and the F-words for childhood development. Paediatr. Child Health 2021, 31, 352–358. [Google Scholar] [CrossRef]
- Gibson, B.E.; Teachman, G.; Wright, V.; Fehlings, D.; Young, N.L.; McKeever, P. Children’s and parents’ beliefs regarding the value of walking: Rehabilitation implications for children with cerebral palsy. Child Care Health Dev. 2012, 38, 61–69. [Google Scholar] [CrossRef]
- Palisano, R.J.; Shimmell, L.J.; Stewart, D.; Lawless, J.J.; Rosenbaum, P.L.; Russell, D.J. Mobility Experiences of Adolescents with Cerebral Palsy. Phys. Occup. Ther. Pediatr. 2009, 29, 133–153. [Google Scholar] [CrossRef]
- Huang, I.-C.; Sugden, D.; Beveridge, S. Children’s perceptions of their use of assistive devices in home and school settings. Disabil. Rehabil. Assist. Technol. 2009, 4, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Huroy, M.; Behlim, T.; Andersen, J.; Buckley, D.; Fehlings, D.; Kirton, A.; Pigeon, N.; Mishaal, R.A.; Wood, E.; Shevell, M.; et al. Stability of the Gross Motor Function Classification System over time in children with cerebral palsy. Dev. Med. Child Neurol. 2022, 64, 1487–1493. [Google Scholar] [CrossRef] [PubMed]
- Demers, L.; Weiss-lambrou, R.; Ska, B. The Quebec User Evaluation of Satisfaction with assistive Technology (QUEST 2.0): An overview and recent progress. Technol. Disabil. 2002, 14, 101–105. [Google Scholar] [CrossRef] [Green Version]
- Murchland, S.; Kernot, J.; Parkyn, H. Children’s Satisfaction With Assistive Technology Solutions for Schoolwork Using the QUEST 2.1: Children’s Version. Assist. Technol. 2011, 23, 162–176. [Google Scholar] [CrossRef]
- Demers, L.; Weiss-Lambrou, R.; Ska, B. Item Analysis of the Quebec User Evaluation of Satisfaction with Assistive Technology (QUEST). Assist. Technol. 2000, 12, 96–105. [Google Scholar] [CrossRef]
- Field, D.A.; Livingstone, R.W. Parents’ and Therapists’ Satisfaction with Four Early Childhood Power Mobility Devices. Can. J. Occup. Ther. 2022, 89, 364–375. [Google Scholar] [CrossRef]
- Tefft, D.; Guerette, P.; Furumasu, J. The Impact of Early Powered Mobility on Parental Stress, Negative Emotions, and Family Social Interactions. Phys. Occup. Ther. Pediatr. 2011, 31, 4–15. [Google Scholar] [CrossRef]
- Gudjonsdottir, B.; Gudmundsdottir, S.B. Mobility devices for children with physical disabilities: Use, satisfaction and impact on participation. Disabil. Rehabil. Assist. Technol. 2021, 1–8. [Google Scholar] [CrossRef]
- Fox-Hustwaite, M. What moves you? Teaching mobility versus developing motor skills based on the developmental continuum. Rehab. Manag. 2013, 26, 36–41. [Google Scholar]
- Russell, D.J.; Gorter, J.W. Assessing functional differences in gross motor skills in children with cerebral palsy who use an ambulatory aid or orthoses: Can the GMFM-88 help? Dev. Med. Child Neurol. 2005, 47, 462–467. Available online: http://www.ncbi.nlm.nih.gov/pubmed/15991866 (accessed on 11 December 2022). [CrossRef]
- van der Putten, A.; Vlaskamp, C.; Reynders, K.; Nakken, H. Movement skill assessment in children with profound multiple disabilities: A psychometric analysis of the Top Down Motor Milestone Test. Clin. Rehabil. 2005, 19, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Law, M.; Baptiste, S.; McColl, M.; Opzoomer, A.; Polatajko, H.; Pollock, N. The Canadian Occupational Performance Measure: An Outcome Measure for Occupational Therapy. Can. J. Occup. Ther. 1990, 57, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Gorter, J.W.; Ketelaar, M.; Rosenbaum, P.; Helders, P.J.M.; Palisano, R. Use of the GMFCS in infants with CP: The need for reclassification at age 2 years or older. Dev. Med. Child Neurol. 2009, 51, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Palisano, R.J.; Cameron, D.; Rosenbaum, P.L.; Walter, S.D.; Russell, D. Stability of the Gross Motor Function Classification System. Dev. Med. Child Neurol. 2006, 48, 424–428. [Google Scholar] [CrossRef]
- Hanna, S.E.; Bartlett, D.J.; Rivard, L.M.; Russell, D.J. Reference Curves for the Gross Motor Function Measure: Percentiles for Clinical Description and Tracking Over Time Among Children With Cerebral Palsy. Phys. Ther. 2008, 88, 596–607. [Google Scholar] [CrossRef]
- Dumas, H.M.; Fragala-Pinkham, M.A.; Moed, R. Scoping Review of Judgment-Based Measures of Ambulation with Assistive Devices for Children and Youth. Phys. Occup. Ther. Pediatr. 2021, 41, 120–137. [Google Scholar] [CrossRef]
- Chagas, P.S.C.; Magalhães, E.D.D.; Junior, R.R.S.; Romeros, A.C.S.F.; Palisano, R.J.; Leite, H.R.; Rosenbaum, P. Development of children, adolescents, and young adults with cerebral palsy according to the ICF: A scoping review. Dev. Med. Child Neurol. 2022, 1–9. [Google Scholar] [CrossRef]



| Citation | Purpose | Relevant Studies | Conclusions |
|---|---|---|---|
| Amacher- Steiner, 2010 [51] | Review evidence for supported standing in relation to occupational therapy theory | Kuenzle [60], Eisenberg [17], Wright [61] | Dynamic standers (SSDs) positively impact mobility and hand function. Prescription is supported by occupational therapy theory. |
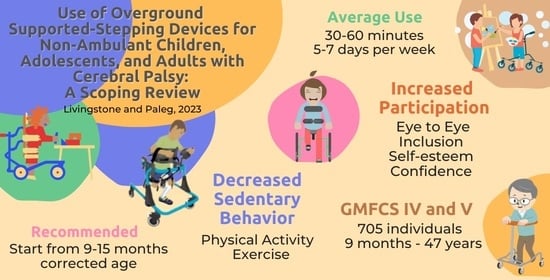
| Paleg, 2015 [15] COI reported Unfunded | Examine effect of SSDs on BSF, Activity or Participation outcomes for children up to 18 years | Barnes [67], Broadbent [75], Eisenberg [17], Farrell [93], Lancioni [69,70,71,72,73,74], McKeever [64], Van der Putten [59], Whinnery [101], Willoughby [58], Wright [18,61] | Youngest reported: 2–3 years; mean: 10 years. Recommended starting at 9–12 months. Mainly positive evidence, but primarily descriptive and insufficient to draw strong conclusions. Trend to increased walking distance and number of steps. Significant increase in mobility level and improved bowel function. Association between increased weight-bearing and bone mineral density. Positive impact on affect, motivation, and participation. |
| Paleg, 2016 [19] COI reported Unfunded | To review evidence and clinical considerations for selection of SSD features | 6 interventions [17,18,61,64,75,93], 3 devices [113,114,115], 1 device properties [116], 3 expert opinions [80,92,96], 1 survey [88] | Evidence supporting selection of SSDs and/or SSD features is descriptive. Research on all aspects of SSD development and prescription is needed. |
| Livingstone, 2016 [25] COI reported Unfunded | To review evidence supporting outcome measures that have been used, or may be useful for SSD outcomes | Barnes [67], Eisenberg [17], Farrell [93], Lancioni [69,70,71], Van der Putten [59], Willoughby [58], Wright [18,61] | Functional and individualized measures are more useful than spatio-temporal measures for GMFCS IV/V. Outcome measures for this population and intervention require development. |
| Booth, 2018 [52] Independent grant | Effectiveness of functional gait training in improving gait in children with CP | Willoughby [58], Farrell [93] | Functional gait training is safe and more effective for improving walking speed than standard physical therapy. Only interventions with partial body weight support are suitable for GMFCS IV/V. |
| Novak, 2020 [53] No COI | Summarize best available evidence for managing CP in 2019 | Paleg, 2015 [15] | Low quality evidence supports use of SSDs to increase gross-motor function and ability to step for GMFCS IV/V. Weak + recommendation. |
| Paleg, 2021 [54] COI reported Unfunded | Review use of postural management interventions to promote hip health in children and adults with non-ambulatory CP | Paleg, 2015 [15] | No studies have examined impact of SSDs on hip health. Clinical recommendation: active weightbearing and movement in SSD to promote position change and reduce sedentary behavior. |
| Gannotti, 2021 [55] Declared no COI Independent grant | Exercise interventions for bone health: guidelines for individuals with CP | N/A | Overground fast walking in SSD may provide moderate osteogenic exercise for GMFCS IV. Active assisted movement opportunities may benefit GMFCS V. |
| Jackman, 2022 [56] Declared no COI Independent grant | Clinical guideline: interventions to improve physical function in children with CP | Novak, 2020 [53], Booth, 2018 [52] | Overground more effective than partial body-weight supported treadmill training to increase walking distance for GMFCS IV/V. SSDs used for inclusion and well-being rather than functional mobility. |
| de Campos, 2023 [57] COI reported Unfunded | Scoping review: early interventions for young children GMFCS IV/V | Jackman [56], Eisenberg [17] | SSDs positively impact functioning, fitness, fun, friends, family and future for children under 5 years who are non-ambulant. |
| Citation | Participants | Intervention | Results/Findings |
|---|---|---|---|
| Source | Randomized controlled group design (RCT) | ||
| Willoughby et al., 2009 [58] Peer-reviewed MMAT 4/5 Declared no COI Independent funding | Controls: 11 GMFCS IV 3 GMFCS III 11.24 ± 4.17 years Participants: 7 GMFCS IV 5 GMFCS III 10.35 ± 3.14 years | 30 min 2 times per week for 9 weeks Controls: Overground walking with anterior support-arms SSD # (GMFCS IV) or posterior walker # (GMFCS III) Participants: Partial body-weight supported treadmill training (PBWSTT) | No statistically significant between-group differences in: endurance (10 Minute Walk Test); or speed (10 Meter Walk Test). Trend to increased walking distance and endurance favoring overground training. Trend to decreased walking distance in PBWSTT group. Conclusion: to improve overground walking, practice overground. |
| Non-randomized group designs | |||
| Eisenberg et al., 2009 [17] Peer-reviewed MMAT 4/5 COI and funding not reported | Participants: 11 GMFCS IV/V 6.1 ± 2.1 years Controls: 11 GMFCS IV/V 6.8 ± 1.7 years | Use over 6 months: Participants: Hands-free orthotic SSD 2.1 ± 1.8 increased to 4.5 ± 2.3 h per week Controls: Stander 2.1 ± 1.8 h per week | Participants: Increased PEDI mobility (p = 0.03). Decreased constipation (p = 0.02). Increased walking distance. Participants and Controls: Moderate association between increased BMD and increased weightbearing in stander or SSD. |
| van der Putten et al., 2005 [59] Peer-reviewed MMAT 3/5 COI and funding not reported | Participants: 32 GMFCS IV/V Mean 8.8 years Controls: 12 GMFCS IV/V Mean 10.6 years | Participants: 12 months MOVE® program including use of anterior support-arms SSD with sling seat Controls: 12 months regular education and therapy program | Both groups increased movement skill independence on TDMMT. MOVE®: 19.2 ± 13.5 to 22.8 ± 14.5. Controls: 20.2 ± 16.3 to 22.3 ± 19.2. Significant increase (p < 0.001) with moderate ES (0.69): MOVE® group. 4/12 controls improved. 20/32 MOVE® group improved. |
| Pre-post single group designs | |||
| Kuenzle and Brunner, 2009 [60] Peer-reviewed MMAT 4/5 Declared no COI Distributor involved in study conduct and planning | 45 GMFCS IV 48 GMFCS V Mean 7.6 years (1.8–18 years) | Hands-free orthotic SSD 88/93 completed 3-month evaluation: 1 deceased, 4 returned SSD Mean use: 0.8 h (0.2 to 2.5) day, 5 times per week (range 2–7) | 78 walked with little/no assistance; 10 used for dynamic standing only. Distance: 98.5 m ± 11.06 (2–463 m). Significant increase in walking abilities (WeeFIM) p < 0.001. Parents rated significantly increased mobility independence compared with no SSD (p < 0.001), but only hand function improved compared with previous device (p < 0.001). Goals for SSD met or exceeded: 81% parents and 86% therapists. Contraindications: hip/knee flexion contractures > 20 degrees; ankle dorsiflexion < 0 degrees. |
| Smati et al., 2022 [62] Peer-reviewed MMAT 4/5 Declared no COI Independent funding | 4 GMFCS III 5 GMFCS IV 6–11 years | 12-week, 50 min, 3 times per week intensive power training during school PE GMFCS IV used SSDs GMFCS III used walkers | Results for GMFCS IV only: HR/min 1.57 ± 0.72 to 0.95 ± 0.53. 10 MWT 15.62 ± 10.1 to 11.86 ± 5.65 s. 10 MFWT 10.32 ± 3.14 to 8.64 ± 3.42 s. 50 MST 67.4 ± 34.63 to 50.4 ± 17.78 s. 6-min walk endurance 162.75 ± 44.46–269.77 ± 88.05 m. Greatest change: children 6, 7, 8 yrs. 10, 11 yr. old results relatively stable. |
| Wright and Jutai, 1999 [18] Peer-reviewed MMAT 4/5 Independent funding | 5 GMFCS IV 15 GMFCS V 7.9 ± 2.9 years | Hands-free orthotic SSD for 12 months Initial use: 30 min daily Mean use from 2–12 months: 322 min ± 190.5 per week (range 92.5–860) | Significant increase (p = 0.006) in GMFM walk run jump (WRJ) score in SSD from 2–12 months: 6.1 ± 6.1. DMA significant increase (p = 0.01) from 2–12 months: 6.6 ± 10.7. Significantly increased walking speed and distance (p < 0.05). PEDI mobility score increased mean 10% points (p = 0.04) with SSD introduction, then remained stable. PEDI social function increased 6% points ± 8.5 by 12 months. 6 children walked indoors and out; 6 within home and classroom; 7 used for standing and exercise. |
| Wright and Jutai, 2006 [61] Peer-reviewed MMAT 3/5 Independent funding | 9 GMFCS III 10 GMFCS IV Mean age at 36-month follow-up 10.7 ± 3.1 years 12 children > 12 years | Hands-free orthotic SSD | 13/20 still used as main SSD. 6/7 who discontinued were >12 years and had outgrown the device. For 12/13 still using this SSD: non-significant increases (<3% points) in GMFM stand or WRJ. DMA: significant increase in steering ability between 12 and 36 months: 12 to 28.6 (p = 0.02). Walking speed unchanged. PEDI caregiver assistance scale: mean gains in self-care (4; p = 0.52) and social function (6; p = 0.04) scales from 24–36 months. |
| Qualitative | |||
| Bradbury and Tierney, 2022 [63] Peer-reviewed MMAT 4/5 Declared no COI Independent funding | Parent of 1 GMFCS IV 10 PTs (Excluded 7 children and 5 parents at GMFCS II/III) | 1 GMFCS IV: ring style SSD PTs discussed varied SSDs | Child safety, comfort, and happiness influence parent use of SSDs. PTs split between valuing child function and participation and promoting gait pattern. Recommendation: prioritize participation and child/family goals. |
| McKeever et al., 2013 [64] Peer-reviewed MMAT 5/5 Independent funding | Parents of children 10 GMFCS IV 9 GMFCS III Mean 10.7 years ± 3.1 years | Hands-free orthotic SSD Parents interviewed 36 months after provision. 13 children still using this specific SSD | Theme 1. Bodily function, position, and comportment. Theme 2. Communication. Theme 3. Participation and inclusion. Theme 4. Freedom and independence. Parents report child appeared more able. They valued SSD for enabling social inclusion in daily life as much as enhanced independent mobility. |
| Rodriguez Costa et al., 2021 [65] Peer-reviewed MMAT 5/5 Declared no COI Independent funding | 2 parents, 2 educational professionals and 4 PTs interviewed regarding experiences using the low-cost hands-free SSD with four children at GMFCS IV # Mean age 8.75 ± 5.5 years at time of study Children started using SSD at age 3–6 years | Low-cost charity developed Hands-free SSD used for 2.5 ± 1 years | Theme 1. Emotional welfare: happiness, emotional regulation, and self-esteem. Theme 2. Physical well-being: improved fitness, bowel function, balance and walking. Theme 3. Social Enjoyment: integration with others, increased hand-use, and independent exploration. Conclusion: Using the SSD made the children happier, increased self-confidence and autonomy, and enhanced participation. |
| Mixed methods | |||
| Paananen, 2009 [66] Thesis MMAT 4/5 | GMFCS IV/V * 7 aged 3–6 years 5 aged 7–15 years | Hands-free orthotic SSD 7 used 4–6 months 1 used 7–9 months 4 used > 12 months Used 30 min to 2 h daily | Parents satisfied (score 4) or very satisfied (score 5) (QUEST 2.0). Device satisfaction 4.6 ± 0.93. Services satisfaction 4.02 ± 1.01. Most important factors: Effective: Satisfied (2/12) or very satisfied (10/12). Ease of use: only 5/12 satisfied. Comfort: Satisfied (3/12) or very satisfied (9/12). Children were motivated to use. 7/12 used for both standing and walking. Used indoors and out. Increased participation in family life and with other children at school. Physical and attention benefits. Negatives: difficulty with transfers. |
| Single-subject research: Multiple baseline design | |||
| Barnes et al., 2002 [67] Peer-reviewed | Case 3: 3.5 years; GMFCS V * Case 4: 9 years GMFCS IV * Excluded participants: Cases 1 and 2: Independent walking Case 5: did not use SSD | Anterior support-arms SSD with sling seat, as part of MOVE® intervention over 8–9 months: stepping data collected twice weekly Case 3: 15-week baseline; 37-week intervention Case 4: 20-week baseline; 32-week intervention Two years maintenance data | Case 3: Initially unable to weight-bear unless fully supported in stander. Unable to step in SSD or with adult support during baseline or intervention. Able to walk 100 ft in SSD in maintenance phase. Case 4: Progressed to walking 70 ft in SSD; 9 min 20 s after 30 trials; 4 min 54 s by trial 53. Child unavailable in maintenance phase. |
| Lancioni, 2005a [68] Peer-reviewed | GMFCS IV/V * Profound intellectual disability Case 1: 10.8 years Case 2: 47.7 years | Straddle style SSD with chest support, saddle, and foot divider. Modified with automatic step counter and microswitch technology to activate preferred stimuli on stepping. 5-min sessions, 3–4 times daily, 4–6 times per week | Steps counted in baseline (Case 1: 6; Case 2: 24 sessions) but preferred stimuli (e.g., music, lights, vibration) activated only during intervention. Significant increase (p < 0.01) in steps (Case 1: 24 to 103; Case 2: 70 to 194) and indices of happiness: (Case 1: 1 to 5; Case 2: 1 to 7) during intervention phases for both. |
| Single-subject research designs: ABAB | |||
| Lancioni et al., [69,70,71,72,73,74] Peer-reviewed 2005b [69] 2007a [70] 2007b [71] 2008 [72] 2010 [73] 2013 [74] | GMFCS IV/V *; 3 to 43 years; Mean 13.26 ± 10.26 years; Profound intellectual disability; 9/17 severe visual impairment Case 1: 13 years; 1 month [69] Cases 2, 3: 10 years; 8 years [70] Cases 4–7: 6.7 years; 8.9 years; 19.2 years; 41.2 years [71] Case 8: 3 years (AB only) [72] Case 9: 12 years [72] Cases 10–14: 5.6 years; 6.5 years; 7.2 years; 11.4 years; 10.1 (AB only) years [73] Cases 15–17: 10.5 years; 12 years; 34 years [74] | Ring walker SSD with saddle or sling seat Modified with automatic step counter and microswitch technology to activate preferred stimuli Intervention: 4.7 ± 0.82 (2–5) minutes 7.6 ± 7.74 (4–28) times per day Total sessions in each intervention phase: 133.6 ± 171.82 (33 to 873) | Significant increases (p < 0.01) in stepping, pushing or leg-foot movements in SSD and indices of happiness during intervention phases, with change of slope towards baseline during withdrawal phase. Cases 4 and 5: stepping significant at p < 0.05. |
| Case series and observational studies | |||
| Broadbent et al., 2000 [75] Peer-reviewed | GMFCS IV * Case 1: 9 years Case 2: 14.5 years Case 3: 8 years Case 4: 8 years | Study-specific hands-free orthotic SSD 1. 30 min 3 times per week 2. 2 h 4 times per week 3. 4 h 5 times per week 4. 40 min 2–4 times per week | Independent walking increased (body weight support %). 1. >400 m indoor/outdoor (37%). 2. 10–25 m indoors (81%). 3. >400 m indoor/outdoor (69%). 4. 100–400 m outdoors (63%). |
| Camallonga, 2010 [76] Thesis | 10 children, aged 1–4 years. 6 GMFCS IV 4 GMFCS V Age 18–52 months; children determined not to be candidates: 3 GMFCS IV, 3 GMFCS V; Level of Sitting Scale (LSS): LSS 2:1; LSS 3:3; LSS 8:1 Developmental age: 3 weeks to 3 months, 3 weeks | Hands-free orthotic SSD used in early intervention setting | Case 1: 29 months, GMFCS IV, LSS 4, dev. age 11 months. Case 2: 21 months, GMFCS IV, LSS 4 dev. age 7 months, 2 weeks. Case 3: 38 months, GMFCS V, LSS 3, dev. age 6 months. Case 4: 44 months, GMFCS IV, LSS 4, dev. age 18 months. Protocol for successful candidates: LSS 2–4; GMFCS IV/V; developmental age 6 months. 3/4 stepping to explore classroom. 1/4 mainly standing. All increased motivation, self-expression. |
| Gandarias Mendieta, 2012 [77] Conference paper Device distributor | 126 children < 18 years GMFCS IV/V 11 adults GMFCS IV/V 18–37 years | Longitudinal follow-up: hands-free orthotic SSD | GMFCS III: this SSD type limits their autonomy and function. GMFCS IV: achieve most functional stepping, indoor and outdoor use. GMFCS V: improved posture, head, and saliva control. Important for self-esteem. May use for dynamic standing and some can step. |
| Gandarias Mendieta, 2023 [86] Unpublished longitudinal clinical database, all new device users in Spain provided by author 16 January 2023 Device distributor | 40 GMFCS III/other conditions 105 GMFCS IV 180 GMFCS V Total GMFCS IV/V: 285 Includes population from previously reported 137 users [77] and 26 users from two independent studies [81,82] Contact lost: 27 users Deceased: 6 GMFCS V, 3 others | Longitudinal follow-up of hands-free orthotic SSD use from 2010 to 2022 Age: 12 months to 37 years Average age of introduction: 3–6 years Average use: 7 years GMFCS IV: 96 <18 years; 9 adults/105 GMFCS V: 142 <18 years; 38 adults/180 | Current users as of December 2022: 37 GMFCS IV and 74 GMFCS V. Aged 2–34 years: mean 13 years. Able to step: 32 GMFCS IV and 63 GMFCS V. Using other SSD: 37 GMFCS IV and 4 GMFCS V. No longer using due to poor health: 5 GMFCS IV and 42 GMFCS V. Outgrown size: 33 GMFCS IV and 48 GMFCS V. |
| Livingstone et al., 2023 [78] Manuscript under review COI reported Unfunded | 8 GMFCS III 15 GMFCS IV 19 GMFCS V 47.74 months ± 14.71 months | SSD use over 6 months Baseline: III:6/8 IV:13/15 V:13/19 6 months: III: 8/8 IV:14/15 V:14/19 | SSD type according to GMFCS: III: 6 Convertible; 2 Support-arms. IV: 9 Support-arms; 5 Hands-free. V: 12 Hands-free; 2 Support-arms. No significant change in SSD use over 6 months. Power mobility introduction did not reduce SSD use at any GMFCS level. |
| Low, 2005 [79] Conference abstract | 39 severe multiple disabilities >50% GMFCS IV/V * Mean 9.2 years (3.5–13 years) | Anterior support-arms SSD with sling seat as part of MOVE® program over 1 year | 31/39 increased mobility level 1/39 independent walking. Increased independence in sitting, standing, and walking for all ages. Increased alertness and ease-of-care. |
| Low et al., 2011 [80] Conference abstract | 9 GMFCS IV 1 GMFCS V 7.5 ± 3.3 years | Comparison of *anterior support-arms SSD and *posterior hands-free SSD after 1 week trial in each | No significant group differences in gait speed/quality. Both SSDs improved upright mobility. For 6/10 new to SSDs, walking speed/gait quality better in hands-free SSD. 6 families preferred anterior. SSD: ease of transfers and use. |
| Martin Gomez et al., 2012 [81] English translation Manufacturer web publication | 7 GMFCS IV 19 GMFCS Mean 10.2 years | Hands-free orthotic SSD Mean use: 8.5 h per week for 25.5 months | Typical user is GMFCS V. 20/26 parents were very satisfied. QUEST 2.1 mean score 4.29 (2.4–6.3). |
| Martin Gomez et al., 2021 [82] Conference abstract | 19/26 GMFCS IV/V followed longitudinally since 2012 analysis | Hands-free orthotic SSD Mean use 5.2 years Range 2–9 years | 1/19 still using device. Reasons for discontinuation: increased deformity (13); size (3); improved abilities (2); time (1). QUEST 2.1 mean 3.12 (1.2–5.3). |
| Paleg, 1997 [83] Conference abstract | 19 GMFCS IV/V # Unable to sit, stand or walk Mean 6 years (2–14) | Anterior support-arms SSD as part of one-week intensive in-patient 20-h program based on MOVE® | 18/19 children able to reciprocally step more than 19 feet in SSD. 11/19 maintain head control 30 s in upright supported sitting. 8/19 maintain hip and knee extension 30 s. |
| Sharma and Bajracharya., 2022 [84] Peer reviewed Declared no COI Funding declared | 5 GMFCS II-IV Results here reported for: 3 GMFCS IV # Case 1: 8 years Case 2: 7 years Case 3: 6 years | Examined feasibility and parent satisfaction with a low-cost hands-free SSD: modified standard 4-wheel reverse walker by adding circumferential trunk support and sling seat | # Parents quite/very satisfied: QUEST 2.0 total 4.85 ± 0.25. Most important items: dimensions: 4.85 ± 0.25; ease of use: 5/5; effectiveness: 4 ± 1. All able to take a few steps hands-free. Case 1 able to play ball games and drink independently in SSD. Modification: feasible option for lower-resourced settings. |
| Wright-Ott, 2018 [85] Web Publication | 29 children 3–10 years 1 GMFCS III 5 GMFCS IV 20 GMFCS V 3 unknown genetic | Hands-free SSDs Preschool aged children; used 30–60 min daily Elementary aged children; used 15 min daily in recess and 30 min 3 times per week in gym and math/science | Teacher, family, and therapist observations: increased interaction with peers, visual attention, self-initiated behavior, problem solving, engagement, upper extremity use, physical motor control and communication. |
| Cross-sectional: Descriptive | |||
| George et al., 2020 [87] Peer-reviewed Declared no COI No funding | UK SSD prescribers (105 PTs, 1 OT, 1 PT aide) and 18 non-prescribers (worked with children using SSDs: PT, OT, education staff) | Prescribers/non-prescribers familiar with a wide range of SSD types. Most commonly prescribed SSDs were: anterior support-arm with sling seat (95/107); anterior-posterior with saddle seat (42/107) and posterior hands-free (38/107). Prescribed use: as much as able (53/107); <30 min (6/107); 30–60 min (34/107); >1 h (14/107) Actual use: <10 min (1/17); 10–30 min (6/17); 30–60 min (6/17); >1 h (4/17) Mean use: 2–5 years (1–10+) | Mean age of introduction 3.6 ± 1.6 years. Youngest age 2.4 ± 1.4 years, dependent on condition, cognition, motivation and motor ability. Conflict between early SSD use as bridge to independent walking or when other options unsuccessful. Contraindications: lack of head control, pain, behavioral problems. Most common reason for discontinuing use: functional deterioration. Challenges: lack of staff, space, or child prefers other mobility method. Top benefits: prescribers: physical activity, enjoyment, participation. Non-prescribers: independence, physical activity, enjoyment. <20% children progress to using hand-held walkers. |
| Low et al., 2011 [88] Peer-reviewed Declared no COI | 513 US pediatric PTs who used SSDs | Commonly prescribed SSDs: anterior support-arms with sling seat (402/513); suspension posterior walker (360/513); anterior support-arms with solid seat (329/513); posterior hands-free (46/513) Time used: <5 h/week (42%); 5–10 h/week (63%); >10 h/week (74%) Duration of use: <6 months (36%); 6 months–1 year (20%); 1–2 years (12%); >2 years (15%) | Factors influencing success: GMFCS level, motivation to walk, and cognition. Commonly recommended to increase exercise. Used in both posterior (65%) and anterior (53%) configurations. PTs consider hip stability, respiratory, and cardiovascular function and BMD when prescribing SSDs. Use of SSDs encouraged through participation with peers and school activities and within ADL activities. 30–50% children progress to walking with hand-held walkers. |
| Peredo et al., 2010 [89] Peer-reviewed Declared no COI | 52 children with CP (GMFCS not reported) as part of survey of medical device use in 108 children with disabilities | Unspecified SSD | 10/52 CP used SSDs, 11/52 used walkers. One other child/108 with a genetic condition used an SSD. 4/11 with SSD never used them, 2 used weekly, and 5 used daily. |
| Roquet et al., 2018 [14] Peer-reviewed Declared no COI Independent funding | 234 GMFCS IV/V 65 aged 2 to 17 years 170 aged 18 to >40 years | Examine change in health-care use for individuals with CP in one region in northern France by age and GMFCS | Use of gait aids (unspecified) is stable but limited. 14–19% across all age groups for GMFCS IV/V. In comparison 68% ≤17 years use standing frames with decline to 16% at 18–24 years and 7% ≥25 years. |
| Case reports | |||
| Altizer and Paleg, 2020 [90] Periodical | GMFCS IV 6 years | Hands-free SSD used daily from 12 months | SSD used to walk with support for exercise. Helps maintain fitness and participation with friends at the playground, store, and at school. Power mobility for longer distances. |
| Benson, 2020 [91] Book chapter | GMFCS IV 4 years | Unspecified SSD used daily in school | Used for free movement within the classroom and for short distances in school. Required power mobility for independence over longer distances. |
| Carnevale, 2015 [92] Conference paper | GMFCS IV 8 years | A. Anterior support-arms SSD B. Posterior hands-free SSD for 12 months C. Anterior SSD with hand holds | Anterior support-arms SSD: bore all weight on arms and dragged feet. Posterior hands-free SSD with full trunk support introduced: Over 12 months, learned to walk in upright position. Anterior orientation re-introduced: able to use hands for steering rather than for support. |
| Farrell et al., 2010 [93] Peer-reviewed Declared no COI | GMFCS IV * 10 years | Anterior support-arms SSD with sling seat 30 min, 3–5 times per week: 4 weeks in-patient program | Able to walk 150 feet with steering assistance. Increased weightbearing, weight shift and decreased hip flexion during stance. Increased GMFM (from 29.62 to 38.45) and PAM scores. Improved standing transfers and bed mobility. |
| George, 2018 [94] Peer-reviewed COI reported | GMFCS IV # 6 years Excluded: cases 2 and 3: not using SSD | # Anterior support-arms SSD with solid seat Stander with side-to-side rocking base 10 min 3–5 times per week for 6 weeks | TDMMT overall, standing, and walking scores improved from level 3 (dependent) to level 2 (walks 10 m with assistance). GAS-Light: consistently initiates and steps continuously over 10 m. Increased reciprocal stepping. |
| Gordon, 2023 [102] Book chapter online video cases | Case 1: GMFCS IV 3 years Case 2: GMFCS V 3 years | Hands-free SSD Daily use from 9 months | Case 1: Participation in family routines, improved hand use and play with siblings. Case 2: Limited active stepping and propulsion with assistance. Increased motivation and participation in gym games with other children in preschool. |
| Jones, 2020 [95] Book chapter | GMFCS IV 3 years | Hands-free SSD | Able to take steps and walk short distances (up to 25 feet) inside the home. Power wheelchair prescribed to keep up with peers outdoors. |
| Kannegießer-Leitner (undated) [103] Manufacturer web publication | Case 1: GMFCS IV/V * 4 years Case 2: GMFCS IV * 22 years Case 3: GMFCS V * 16 years | Hands-free orthotic SSD | Case 1: Improved posture, able to step independently. Increased participation with family. Case 2: Motivated to walk 2–4 km daily. Increased sense of autonomy. Case 3: Enjoys going for a walk outside after school 1.5–2 km daily. |
| Low, 2004 [96] Conference abstract | GMFCS IV * 12 years | Anterior support-arms SSD compared with previously used posterior hands-free SSD | Improved gait quality in anterior SSD. Easier transfers and increased mobility at home and school. |
| Paleg, 1997 [97] Periodical | Spastic diplegia GMFCS IV # 5–9 years | Anterior support-arms SSD with sling seat 4 years MOVE® program | Able to run in SSD, pull to stand, get up from floor with minimal assistance and sit independently. |
| Paleg, 1997 [104] Periodical | Case 1: dystonic quadriplegia GMFCS IV # 5 years Case 2: spastic quadriplegia GMFCS IV # 17 years | Anterior support-arms SSD with sling seat MOVE® program for 1 year Anterior support-arms SSD with sling seat MOVE® program | Able to walk all over school in SSD. He is happy, has friends, is communicating and able to feed himself. Able to take a few independent steps at age 17. Improved speech, bowel function, and self-esteem. |
| Paleg, 1998 [98] Periodical | Holoprosencephaly GMFCS IV # 3 years | SSD, stander, and classroom seat used 20 min each daily for one year | Able to sit to stand with hands held, maintains for 15 s, takes reciprocal steps for 10 feet in SSD. |
| Paleg, 2007 [99] Periodical | Joubert syndrome GMFCS IV # 3 years | Anterior support-arms SSD with solid seat used daily and PBWSTT 8 min 3 times per week from 12 months | 27 months: able to step forward and backwards in SSD with solid seat. 36 months: able to sit to stand and step taking partial body-weight in dynamic anterior sling seat SSD. |
| Pope et al., 2022 [100] Periodical | GMFCS V 5 years | # Anterior support-arms SSD with solid seat | Early upright positioning with stander. SSD for mobility and participation with siblings outdoors. |
| Schneiders (undated) [105] Manufacturer web publication | Several GMFCS V Starting at 18 months | Hands-free orthotic SSD | Alternate between active and static standing. Increased field of view and upright posture changes self-perception and perception of others. Increased self-determination: able to actively approach people or toys or communicate ‘no’ by moving away. Opportunity for motor and socio-emotional development. |
| Schwerin, 2005 [106] Conference abstract | 3 GMFCS IV 4–10 years | Hands-free orthotic SSD for 6 months following botulinum toxin injections to decrease scissoring | Increased trunk control and weightbearing. Improved communication, attention, exploration and independent activity. Caregiver support and space needed for implementation. |
| Thompson, 2005 [107] Book | Case 1: GMFCS IV * 12 years Case 2: GMFCS IV * 6 ½ years | Case 1. Anterior support-arms SSD with sling seat as part of MOVE® program for 2.5 years Case 2. Anterior support-arms SSD with sling seat as part of MOVE® program for 4 years | Case 1: Walks 500 ft in SSD. Increased independence on TDMMT. Increased peer participation. Case 2: Increased independence on TDMMT. Walks length of gym in SSD and practices sit to stand. Stands with two-hand support. Uses power wheelchair with supervision. |
| Whinnery et al., 2002 [101] Periodical | GMFCS IV * 4 years | Anterior support-arms SSD with sling seat daily 6 months MOVE® program | Average daily steps increased from 0 to 125. |
| Citation | Source | Topic | Comments or Recommendations for GMFCS IV or V |
|---|---|---|---|
| Bolton and Donohoe, 2020 [108] | Book chapter | Use of ambulatory assistive devices for children with CP | Can begin trial at 2 years with GMFCS IV/V Not useful for those with persistent flexor withdrawal response. |
| Covert, 2019 [109] | Periodical | Promote pediatric participation with standers, SSDs and wheeled mobility | SSDs can promote physical activity for GMFCS IV and possibly GMFCS V. When prescribing an SSD to promote participation and self-initiated mobility in the community, consider folding and ease of transportation. |
| Gandarias Mendieta, 2008 [110] | Manufacturer website translation of original Spanish language article | Use and benefits of hands-free orthotic SSD | For standing: promotes even weightbearing and slight movement in standing. Peer-level positioning increases participation with other children. For stepping: Promotes reciprocal gait, well-aligned posture, is stable and safe, increases child inclusion, participation and self-esteem. Contraindications: Difficult to turn: not suitable for children able to walk with posterior walker or with hands held. Contractures at hips or knees > 20 degrees. |
| Kannegießer- Leitner (undated) [103] | Manufacturer web publication | Benefits of hands-free orthotic SSD | Increases autonomy and independence for children requiring manual assistance to step. Reciprocating orthosis increases walking distance and reduces caregiver burden. Transfers may be modified for larger children by attaching trunk first, then knees and feet last. Beneficial for hip development. Others perceive child as more able. |
| Marquez, 2019 [111] | Periodical | Promoting function and independence in CP population | SSDs provide more support than walkers and can be used in anterior or posterior orientation. Maximize participation and independence rather than perfecting posture and gait mechanics. |
| Thunberg et al., 2021 [112] | Book chapter | Environmental modifications: for children with developmental motor disorders | Introduce SSDs around 12 months. SSDs promote upright positioning, active stepping and fitness, play, exploration and interaction. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Livingstone, R.W.; Paleg, G.S. Use of Overground Supported-Stepping Devices for Non-Ambulant Children, Adolescents, and Adults with Cerebral Palsy: A Scoping Review. Disabilities 2023, 3, 165-195. https://doi.org/10.3390/disabilities3020012
Livingstone RW, Paleg GS. Use of Overground Supported-Stepping Devices for Non-Ambulant Children, Adolescents, and Adults with Cerebral Palsy: A Scoping Review. Disabilities. 2023; 3(2):165-195. https://doi.org/10.3390/disabilities3020012
Chicago/Turabian StyleLivingstone, Roslyn W., and Ginny S. Paleg. 2023. "Use of Overground Supported-Stepping Devices for Non-Ambulant Children, Adolescents, and Adults with Cerebral Palsy: A Scoping Review" Disabilities 3, no. 2: 165-195. https://doi.org/10.3390/disabilities3020012