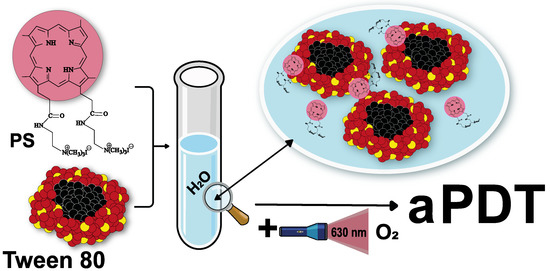
Water-Soluble Dicationic Deuteroporphyrin Derivative for Antimicrobial PDT: Singlet Oxygen Generation, Passive Carrier Interaction and Nosocomial Bacterial Strains Photoinactivation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of PSs
2.3. Spectroscopic Measurements
2.4. Determination of the Quantum Yield of Singlet Oxygen Generation
2.5. Solubility Study
2.6. Determination of Partition Coefficients
2.7. Dynamic Light Scattering Measurements
2.8. Spectrophotometric Titration of PS Aqueous Solutions with Tween 80
2.9. PS Fluorescence Quenching in Aqueous Solutions of Tween 80 in the Presence of KI
2.10. Preparation of PS Solutions and Bacterial Cultures for the Dark and Photoinduced (aPDT) Antibacterial Activity Studies
2.11. Preparation of the Seed Dose of Test Cultures
2.12. aPDT Modeling Technique In Vitro
3. Results and Discussion
3.1. Physicochemical Studies
3.1.1. Spectroscopic Characteristics of Cationic PSs in Organic Solvents
3.1.2. Solvation, Partition and Aggregation of PSs in Aqueous Solutions
3.1.3. PS interaction with Micellar Surfactant Tween 80
3.2. Photoinduced Inactivation of Antibiotic-Resistant Bacterial Microflora
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Banin, E.; Hughes, D.; Kuipers, O.P. Editorial: Bacterial pathogens, antibiotics and antibiotic resistance. FEMS Microbiol. Rev. 2017, 41, 450–452. [Google Scholar] [CrossRef] [Green Version]
- Wainwright, M.; Maisch, T.; Nonell, S.; Plaetzer, K.; Almeida, A.; Tegos, G.P.; Hamblin, M.R. Photoantimicrobials-are we afraid of the light? Lancet Infect. Dis. 2017, 17, e49–e55. [Google Scholar] [CrossRef] [PubMed]
- Amos-Tautua, B.M.; Songca, S.P.; Oluwafemi, O.S. Application of porphyrins in antibacterial photodynamic therapy. Molecules 2019, 24, 2456. [Google Scholar] [CrossRef] [Green Version]
- Hamblin, M.R. Antimicrobial photodynamic inactivation: A bright new technique to kill resistant microbes. Curr. Opin. Microbiol. 2016, 33, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Qin, R.; Zaat, S.A.J.; Breukink, E.; Heger, M. Antibacterial photodynamic therapy: Overview of a promising approach to fight antibiotic-resistant bacterial infections. J. Clin. Translat. Res. 2015, 1, 140–167. [Google Scholar] [CrossRef] [PubMed]
- Algorri, J.F.; Ochoa, M.; Roldán-Varona, P.; Rodríguez-Cobo, L.; López-Higuera, J.M. Photodynamic therapy: A compendium of latest reviews. Cancers 2021, 13, 4447. [Google Scholar] [CrossRef] [PubMed]
- Koifman, O.I.; Ageeva, T.A.; Kuzmina, N.S.; Otvagin, V.F.; Nyuchev, A.V.; Fedorov, A.Y.; Belykh, D.V.; Lebedeva, N.S.; Yurina, E.S.; Surbu, S.A.; et al. Synthesis strategy of tetrapyrrolic photosensitizers for their practical application in photodynamic therapy. Macroheterocycles 2022, 15, 207–305. [Google Scholar] [CrossRef]
- Bonnett, R. Photosensitizers of the porphyrin and phthalocyanine series for photodynamic therapy. Chem. Soc. Rev. 1995, 1, 19–33. [Google Scholar] [CrossRef]
- Zenkevich, E.; Sagun, E.; Knyukshto, V.; Schulga, A.; Mironov, A.; Efremova, O.; Bonnett, R.; Songca, S.P.; Kassem, M. Photophysical and photochemical properties of potential porphyrin and chlorin photosensitizers for PDT. J. Photochem. Photobiol. B 1996, 33, 171–180. [Google Scholar] [CrossRef]
- Dabrowski, J.M.; Arnaut, L.G. Photodynamic therapy (PDT) of cancer: From a local to a systemic treatment. Photochem. Photobiol. Sci. 2015, 14, 1765–1780. [Google Scholar] [CrossRef]
- Sobotta, L.; Skupin-Mrugalska, P.; Piskorz, J.; Mielcarek, J. Porphyrinoid photosensitizers mediated photodynamic inactivation against bacteria. Eur. J. Med. Chem. 2019, 175, 72–106. [Google Scholar] [CrossRef] [PubMed]
- Kustov, A.; Morshnev, P.; Kukushkina, N.; Krestyaninov, M.; Smirnova, N.; Berezin, D.; Kokurina, G.; Belykh, D. The effect of molecular structure of chlorin photosensitizers on photo-bleaching of 1,3-diphenylisobenzofurane—The possible evidence of iodine reactive species formation. Comp. Rend. Chim. 2022, 25, 97–102. [Google Scholar] [CrossRef]
- Suvorov, N.; Pogorilyy, V.; Diachkova, E.; Vasil’ev, Y.; Mironov, A.; Grin, M. Derivatives of natural chlorophylls as agents for antimicrobial photodynamic therapy. Int. J. Mol. Sci. 2021, 22, 6392. [Google Scholar] [CrossRef] [PubMed]
- Kustov, A.V.; Belykh, D.V.; Smirnova, N.L.; Venediktov, E.A.; Kudayarova, T.V.; Kruchin, S.O.; Khudyaeva, I.S.; Berezin, D.B. Synthesis and investigation of water-soluble chlorophyll pigments for antimicrobial photodynamic therapy. Dye. Pigment. 2018, 149, 553–559. [Google Scholar] [CrossRef]
- Kustov, A.V.; Kustova, T.V.; Belykh, D.V.; Khudyaeva, I.S.; Berezin, D.B. Synthesis and investigation of novel chlorin sensitizers containing the myristic acid residue for antimicrobial photodynamic therapy. Dye. Pigment. 2020, 173, 107948. [Google Scholar] [CrossRef]
- Berezin, D.B.; Makarov, V.V.; Znoyko, S.A.; Mayzlish, V.E.; Kustov, A.V. Aggregation water soluble octaanionic phthalocyanines behavior and their photoinactivation antimicrobial effect in vitro. Mend. Commun. 2020, 30, 621–623. [Google Scholar] [CrossRef]
- Van Straten, D.; Mashayekhi, V.; de Bruijn, H.S.; Oliveira, S.; Robinson, D.J. Oncologic photodynamic therapy: Basic principles, current clinical status and future directions. Cancers 2017, 9, 19. [Google Scholar] [CrossRef] [Green Version]
- Kustov, A.V.; Morshnev, P.K.; Kukushkina, N.V.; Smirnova, N.L.; Berezin, D.B.; Karimov, D.R.; Shukhto, O.V.; Kustova, T.V.; Belykh, D.V.; Mal’shakova, M.V.; et al. Solvation, cancer cell photoinactivation and the interaction of chlorin photosensitizers with a potential passive carrier non-ionic surfactant Tween 80. Int. J. Mol. Sci. 2022, 23, 5294. [Google Scholar] [CrossRef]
- Kustov, A.V.; Privalov, O.A.; Strelnikov, A.I.; Koifman, O.I.; Lubimtsev, A.V.; Morshnev, P.K.; Moryganova, T.M.; Kustova, T.V.; Berezin, D.B. Transurethral resection of non-muscle invasive bladder tumors combined with fluorescence diagnosis and photodynamic therapy with chlorin e6-type photosensitizers. J. Clin. Med. 2022, 11, 233. [Google Scholar] [CrossRef]
- Kustov, A.V.; Berezin, D.B.; Zorin, V.P.; Morshnev, P.K.; Kukushkina, N.V.; Krestyaninov, M.A.; Kustova, T.V.; Strelnikov, A.I.; Lyalyakina, E.V.; Zorina, T.E.; et al. Monocationic chlorin as a promising photosensitizer for antitumor and antimicrobial photodynamic therapy. Pharmaceutics 2023, 15, 61. [Google Scholar] [CrossRef]
- Ormond, A.B.; Freeman, H.S. Dye sensitizers for photodynamic therapy. Materials 2013, 6, 817–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yakavets, I.; Millard, M.; Zorin, V.; Lassalle, H.-P.; Bezdetnaya, L. Current state of the nanoscale delivery systems for temoporfin-based photodynamic therapy: Advanced delivery strategies. J. Contr. Release 2019, 304, 268–287. [Google Scholar] [CrossRef] [PubMed]
- Brilkina, A.A.; Dubasova, L.V.; Sergeeva, E.A.; Pospelov, A.J.; Shilyagina, N.Y.; Shakhova, N.M.; Balalaeva, I.V. Photobiological properties of phthalocyanine photosensitizers Photosens, Holosens and Phthalosens: A comparative in vitro analysis. J. Photochem. Photobiol. B Biol. 2019, 191, 128–134. [Google Scholar] [CrossRef]
- Kobayashi, N. Spectroscopically and/or structurally intriguing phthalocyanines and related compounds. Pt 1. Monomeric systems. Chem. Chem. Tech. 2019, 62, 4–46. [Google Scholar] [CrossRef]
- Isakau, H.; Parkhats, M.; Knyukshto, V.; Dzhagarov, B.; Petrov, E.; Petrov, P. Toward understanding the high PDT efficacy of chlorin e6 –polyvinylpyrrolidone formulations: Photophysical and molecular aspects of photosensitizer–polymer interaction in vitro. J. Photochem. Photobiol. B Biol. 2008, 92, 165–174. [Google Scholar] [CrossRef]
- Fontana, L.C.; Pinto, J.G.; Magalhães, J.A.; Tada, D.B.; de Almeida, R.M.S.; Pacheco-Soares, C.; Ferreira-Strixino, J. Comparison of the photodynamic effect of two chlorins, photodithazine and fotoenticine, in gliosarcoma cells. Photochem 2022, 2, 165–180. [Google Scholar] [CrossRef]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.H. Mechanisms in photodynamic therapy: Part three—Photosensitizer pharmacokinetics, biodistribution, tumor localization and modes of tumor destruction. Photodiagnosis Photodyn. Ther. 2005, 2, 91–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khludeev, I.I.; Kozyr’, L.A.; Zorina, T.E.; Zorin, V.P. pH-Dependent changes in the mechanisms of transport of chlorin e6 and its derivatives in the blood. Bull. Exper. Biol. Med. 2015, 160, 208–212. [Google Scholar] [CrossRef]
- Malatesti, N.; Munitic, I.; Jurak, I. Porphyrin-based cationic amphiphilic photosensitisers as potential anticancer, antimicrobial and immunosuppressive agents. Biophys. Rev. 2017, 9, 149–168. [Google Scholar] [CrossRef] [Green Version]
- Berezin, D.B.; Kustov, A.V.; Krestyaninov, M.A.; Batov, D.V.; Kukushkina, N.V.; Shukhto, O.V. The behavior of monocationic chlorin in water and aqueous solutions of non-ionic surfactant Tween 80 and potassium iodide. J. Mol. Liq. 2019, 283, 532–536. [Google Scholar] [CrossRef]
- Pylina, Y.I.; Khudyaeva, I.S.; Startseva, O.M.; Shadrin, D.M.; Shevchenko, O.G.; Velegzhaninov, I.O.; Kukushkina, N.V.; Berezin, D.B.; Belykh, D.V. Dark and photoinduced cytotoxicity of cationic derivatives of chlorin e6 with different numbers of charged groups. Macroheterocycles 2021, 14, 317–322. [Google Scholar] [CrossRef]
- Zhidomorov, N.Y.; Nazarenko, O.A.; Demidov, V.I.; Kustov, A.V.; Kukushkina, N.V.; Koifman, O.I.; Gagua, A.K.; Tomilova, I.K.; Berezin, D.B. Study of acute toxicity of monocationic chlorin e6 derivative, a perspective photosensitizer for antimicrobial and antitumor photodynamic therapy. Biomed. Photonics 2022, 11, 23–32. [Google Scholar] [CrossRef]
- Kruchin, S.O. Synthesis and Physical Chemical Properties of New Dicationic Photosensitizers of Chlorin and Porphyrin Series for Antimicrobial PDT. Ph.D. Thesis, G.A. Krestov Solution Chemistry Institute of Russian Academy of Science, Ivanovo, Russia, 26 November 2020. (In Russian). [Google Scholar]
- Kustov, A.V.; Krestyaninov, M.A.; Kruchin, S.O.; Shukhto, O.V.; Kustova, T.V.; Belykh, D.V.; Khudyaeva, I.S.; Koifman, M.O.; Razgovorov, P.B.; Berezin, D.B. Interaction of cationic chlorin photosensitizers with non-ionic surfactant Tween 80. Mend. Commun. 2021, 31, 65–67. [Google Scholar] [CrossRef]
- Trukhacheva, T.V.; Shlaykhtin, S.V.; Novikov, G.A.; Istomin, Y.A. Fotolon (Photolon)–a New Photosensitizer for Photodynamic Therapy; Belmedpreparaty: Minsk, Russia, 2006; 64p. (In Russian) [Google Scholar]
- Zorina, T.E.; Yankovsky, I.V.; Yakovets, I.V.; Kravchenko, I.E.; Ermilova, T.I.; Shman, T.V.; Belevtsev, M.V.; Zorin, V.P. Intracellular localization and phototoxicity mechanisms of chlorin e6 derivatives and their liposomal formulations. Biophysics 2019, 64, 533–542. [Google Scholar] [CrossRef] [Green Version]
- DiNello, R.K.; Dolphin, D.H. Evidence for a fast (major) and slow (minor) pathway in the Schumm devinylation reaction of vinyl porphyrins. J. Org. Chem. 1981, 46, 3498–3502. [Google Scholar] [CrossRef]
- Falk, J.E. Porphyrins and Metalloporphyrins; Elsevier: Amsterdam, The Netherlands, 1964; 266p. [Google Scholar]
- Tarabukina, I.S.; Startseva, O.M.; Patov, S.A.; Belykh, D.V. Novel dicationic chlorins e6 derivatives. Macroheterocycles 2015, 8, 168–176. [Google Scholar] [CrossRef] [Green Version]
- Kustov, A.V.; Belykh, D.V.; Startseva, O.M.; Kruchin, S.O.; Venediktov, E.A.; Berezin, D.B. New photosensitizers developed on a methylpheophorbide a platform for photodynamic therapy: Synthesis, singlet oxygen generation and modeling of passive membrane transport. Pharm. Anal. Acta 2016, 7, 480–484. [Google Scholar] [CrossRef]
- Schmidt, R.; Afshari, E. Comment on “Effect of solvent on the phosphorescence rate constant of singlet molecular oxygen (1Δg)”. J. Phys. Chem. 1990, 94, 4377–4378. [Google Scholar] [CrossRef]
- Kustov, A.V.; Smirnova, N.L.; Berezin, D.B.; Berezin, M.B. Thermodynamics of solution of proto- and mezoporphyrins in N, N-dimethylformamide. J. Chem. Thermodyn. 2015, 89, 123–126. [Google Scholar] [CrossRef]
- Gerola, A.P.; Tsubone, T.M.; Santana, A.; De Oliveira, H.P.M.; Hioka, N.; Caetano, W. Properties of chlorophyll and derivatives in homogeneous and microheterogeneous systems. J. Phys. Chem. B 2011, 115, 7364–7373. [Google Scholar] [CrossRef]
- Huang, L.; Dai, T.; Hamblin, M.R. Antimicrobial photodynamic inactivation and photodynamic therapy for infections. In Photodynamic Therapy. Methods and Protocols; Gomer, C.J., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 155–174. [Google Scholar]
- Donohoe, C.; Senge, M.O.; Arnaut, L.G.; Gomes-da-Silva, L.C. Cell death in photodynamic therapy: From oxidative stress to anti-tumor immunity. Biochim. Biophys. Acta -Rev. Cancer 2019, 1872, 188308. [Google Scholar] [CrossRef] [PubMed]
- Ishizuka, T.; Grover, N.; Kingsbury, C.J.; Kotani, H.; Senge, M.O.; Kojima, T. Nonplanar porphyrins: Synthesis, properties, and unique functionalities. Chem. Soc. Rev. 2022, 51, 7560–7630. [Google Scholar] [CrossRef] [PubMed]
- Berezin, D.B.; Karimov, D.R. Porphyrins and porphyrin analogs interactions with coordinating organic solvents. Macroheterocycles 2009, 2, 42–51. [Google Scholar] [CrossRef] [Green Version]
- Smith, D.A.; van de Waterbeemd, H.; Walker, D.K.; Mannhold, R.; Kubinyi, H.; Timmerman, H. Pharmacokinetics and metabolism on drug design. In Methods and Principles in Medicinal Chemistry; Mannhold, R., Kubinyi, H., Timmerman, H., Eds.; Wiley–VCH Verlag: Weinheim, Germany, 2001; 141p. [Google Scholar]
- Kustov, A.V.; Smirnova, N.L.; Berezin, D.B.; Berezin, M.B. Blood porphyrins in binary mixtures of N,N-dimethylformamide with 1-octanol and chloroform: The energetics of solvation, solute-cosolvent interactions and model calculations. J. Chem. Thermodyn. 2015, 83, 104–109. [Google Scholar] [CrossRef]
- Kustov, A.V.; Belykh, D.V.; Smirnova, N.L.; Khudyaeva, I.S.; Berezin, D.B. Partition of methylpheophorbide a, dioxidine and their conjugate in the 1-octanol/phosphate saline buffer biphasic system. J. Chem. Thermodyn. 2017, 115, 302–306. [Google Scholar] [CrossRef]
- Lucky, S.S.; Soo, K.S.; Zhang, Y. Nanoparticles in photodynamic therapy. Chem. Rev. 2015, 115, 1990–2042. [Google Scholar] [CrossRef]
- Mironov, A.F.; Zhdanova, K.A.; Bragina, N.A. Nanosized vehicles for delivery of photosensitizers in photodynamic diagnosis and therapy of cancer. Russ. Chem. Rev. 2018, 87, 859–881. [Google Scholar] [CrossRef]
- Gradova, M.A.; Gradov, O.V.; Lobanov, A.V.; Bychkova, A.V.; Nikolskaya, E.D.; Yabbarov, N.G.; Mollaeva, M.R.; Egorov, A.E.; Kostyukov, A.A.; Kuzmin, V.A.; et al. Characterization of a novel amphiphilic cationic chlorin photosensitizer for photodynamic applications. Int. J. Mol. Sci. 2022, 24, 345. [Google Scholar] [CrossRef]
- Kępczyński, M.; Pandian, R.P.; Smith, K.M.; Ehrenberg, B. Do liposome-binding constants of porphyrins correlate with their measured and predicted partitioning between octanol and water? Photochem. Photobiol. 2002, 76, 127–134. [Google Scholar] [CrossRef]
- Abraham, M.H.; Acree, W.E. Solvation descriptors for porphyrins (porphines). New J. Chem. 2016, 40, 9945–9950. [Google Scholar] [CrossRef]
- Giovannetti, R. The use of spectrophotometry UV-Vis for the study of porphyrins. In Nanotechnology and Nanomaterials, Macro to Nano Spectroscopy; Uddin, J., Ed.; InTech: Rijeka, Croatia, 2012; pp. 87–108. [Google Scholar]
- Shukhto, O.V.; Khudyaeva, I.S.; Belykh, D.V.; Berezin, D.B. Aggregation of hydrophobic chlorins with antimicrobial fragments in aqueous solutions of ethanol and Tween 80. Izv. Vyssh. Uchebn. Zaved. Khim. Khim. Tekhnol. 2021, 64, 86–96. [Google Scholar] [CrossRef]
- Batov, D.V.; Kustov, A.V.; Kruchin, S.O.; Makarov, V.V.; Berezin, D.B. Aggregation of cationic chlorin e6 derivatives in water and aqueous solutions of polyvinilpyrrolidone. J. Struct. Chem. 2019, 60, 443–448. [Google Scholar] [CrossRef]
- Chin, W.W.; Heng, P.W.; Lim, P.L.; Lau, W.K.; Olivo, M. Improved formulation of photosensitizer chlorin e6 polyvinylpyrrolidone for fluorescence diagnostic imaging and photodynamic therapy of human cancer. Eur. J. Pharm. Biopharm. 2008, 69, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Schwartzberg, L.S.; Navari, R.M. Safety of polysorbate 80 in the oncology setting. Adv. Ther. 2018, 35, 754–767. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, M.E.; Al-Koofee, D.A.F. Effect of temperature changes on critical micelle concentration for Tween series surfactant. Global J. Sc. Front. Res. Chem. 2013, 13, 1–7. [Google Scholar]
- Pereira, M.A.; Faustino, M.A.; Tome, J.P.; Neves, M.G.; Tomé, A.C.; Cavaleiro, J.A.; Cunha, A.; Almeida, A. Influence of external bacterial structures on the efficiency of photodynamic inactivation by a cationic porphyrin. Photochem. Photobiol. Sci. 2014, 13, 680–690. [Google Scholar] [CrossRef] [PubMed]
- Drulis–Kawa, Z.; Bednarkiewicz, A.; Bugla, G.; Stręk, W.; Doroszkiewicz, W. Bactericidal effects of the Fotolon (chlorin e6) on Gram-negative and Gram-positive strains isolated from wound infections. Adv. Clin. Exp. Med. 2006, 15, 279–283. [Google Scholar]
- Hamblin, M.R. Potentiation of antimicrobial photodynamic inactivation by inorganic salts. Exp. Rev. Anti-Infect. Ther. 2017, 15, 1059–1069. [Google Scholar] [CrossRef]
- Vieira, C.; Gomes, A.T.P.C.; Mesquita, M.Q.; Moura, N.M.M.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Almeida, A. An insight into the potentiation effect of potassium iodide on aPDT efficacy. Front. Microbiol. 2018, 9, 2665. [Google Scholar] [CrossRef] [Green Version]
- Koifman, O.I.; Askarov, K.A.; Berezin, B.D.; Enikolopyan, N.S. Natural sources of porphyrins. Methods for the isolation and modification of natural porphyrins. In Porphyrins: Structure, Properties, Synthesis; Enikolopyan, N.S., Ed.; Nauka: Moscow, Russia, 1985; pp. 175–204. (In Russian) [Google Scholar]






| Parameter | Comp. 5 | Comp. 6 | Comp. 7 | |||
|---|---|---|---|---|---|---|
| Singlet oxygen quantum yield, γΔa | ||||||
| - 0.71 (C6H6) | 0.60 b [14] - | 0.67 b [14] 0.61 (C6H6) [14] | ||||
| Aqueous solubility and partition coefficient | ||||||
| T, K | Solubility, mg/mL | P | Solubility, mg/mL | P [14] | Solubility, mg/mL | P [19] |
| 298.15 | 0.120 ± 0.017 | 0.25 ± 0.03 | 0.038 ± 0.004 | 1.04 ± 0.02 | >5 | 1.88 ± 0.06 |
| 308.15 | 0.135 ± 0.006 | 0.14 ± 0.07 | 0.046 ± 0.004 | 1.36 ± 0.03 | - | 1.90 ± 0.09 |
| 318.15 | 0.167 ± 0.023 | 0.11 ± 0.02 | 0.054 ± 0.003 | 1.61 ± 0.03 | - | 1.91 ± 0.10 |
| Parameter | Comp. 5 | Comp. 6 [34] | Comp. 7 [18] |
|---|---|---|---|
| Equation (3) | |||
| mT1, mol/kg | (0.37–1.81)·10−4 | (3.9–14.6)·10−4 | (0.18–1.7)·10−4 |
| mT2, mol/kg | (2.35–5.48)·10−4 | (3.1–5.5)·10−3 | (1.7–6.7)·10−4 |
| n1 | 1.08 ± 0.02 | 1.76 ± 0.09 | 0.71 ± 0.07 |
| n2 | 2.22 ± 0.27 | 3.26 ± 0.28 | 2.18 ± 0.30 |
| log(Kb)1 | 4.94 ± 0.09 | 5.66 ± 0.29 | 3.73 ± 0.31 |
| log(Kb)2 | 9.30 ± 0.90 | 9.09 ± 0.67 | 9.36 ± 1.04 |
| Equation (4) | |||
| KSV, kg/mol | 10.92 ± 0.30 (60) 1 | 7.78 ± 0.08 (60) | 2.62 ± 0.03 (60) |
| Pathogen, Initial Number of CFU × 107 | |||
|---|---|---|---|
| Escherichia coli | Enterobacter cloacae | Klebsiella pneumonia | |
| Darkness | |||
| PS + 0.5 wt% Tween 80 | 107 | 107 | 0 |
| PS + 0.1 wt% Na2H2Edta | 5 | 0 | 5 |
| PS + 0.001 wt% H2O2 | 107 | 107 | 2 |
| “Fotolon” 1 | 105 | - | 105 |
| Dose 40 J/cm2 | |||
| PS + 0.5 wt% Tween 80 | 107 | 107 | 0 |
| PS + 0.1 wt% Na2H2Edta | 5 | 0 | 0 |
| PS + 0.001 wt% H2O2 | 0 | 107 | 0 |
| Dose 80 J/cm2 | |||
| PS + 0.5 wt% Tween 80 | 0 | 107 | 0 |
| PS + 0.1 wt% Na2H2Edta | 0 | 0 | 0 |
| PS + 0.001 wt% H2O2 | 0 | 3·106 | 0 |
| “Fotolon” 1 | 5 × 102 | - | 103 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berezin, D.B.; Kruchin, S.O.; Kukushkina, N.V.; Venediktov, E.A.; Koifman, M.O.; Kustov, A.V. Water-Soluble Dicationic Deuteroporphyrin Derivative for Antimicrobial PDT: Singlet Oxygen Generation, Passive Carrier Interaction and Nosocomial Bacterial Strains Photoinactivation. Photochem 2023, 3, 171-186. https://doi.org/10.3390/photochem3010011
Berezin DB, Kruchin SO, Kukushkina NV, Venediktov EA, Koifman MO, Kustov AV. Water-Soluble Dicationic Deuteroporphyrin Derivative for Antimicrobial PDT: Singlet Oxygen Generation, Passive Carrier Interaction and Nosocomial Bacterial Strains Photoinactivation. Photochem. 2023; 3(1):171-186. https://doi.org/10.3390/photochem3010011
Chicago/Turabian StyleBerezin, Dmitry B., Sergey O. Kruchin, Natal’ya V. Kukushkina, Evgeny A. Venediktov, Mikhail O. Koifman, and Andrey V. Kustov. 2023. "Water-Soluble Dicationic Deuteroporphyrin Derivative for Antimicrobial PDT: Singlet Oxygen Generation, Passive Carrier Interaction and Nosocomial Bacterial Strains Photoinactivation" Photochem 3, no. 1: 171-186. https://doi.org/10.3390/photochem3010011