Application of Zirconia/Alumina Composite Oxide Ceramics as Photocatalysts for Removal of 2,4,6-Trichlorophenol from Water
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
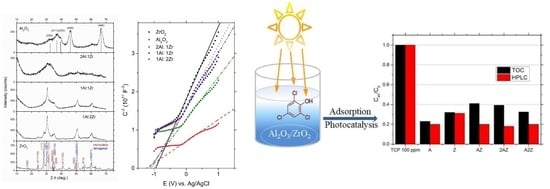
3.1. Structural and Morphological Properties of Oxide Samples
3.2. Textural Properties of Pure and Composite Samples
3.3. Optical Properties of Pure and Composite Oxide Samples
3.4. Determination of Flat Band Potentials of Oxide Samples (Mott–Schottky Plots)
| Sample | Efb (V) vs. SHE 1 | ECB(V) | EVB(V) | Band Gap Energy (eV) |
|---|---|---|---|---|
| Al2O3 | −1.04 | −1.14 | 2.76 | 3.9 |
| 2Al2O3–1ZrO2 | −0.91 | −1.05 | 2.75 | 3.8 |
| 1Al2O3–1ZrO2 | −0.88 | −1.00 | 3.10 | 4.1 |
| 1Al2O3–2ZrO2 | −0.78 | −0.92 | 3.58 | 4.5 |
| ZrO2 | −0.74 | −0.94 | 3.96 | 4.9 |
3.5. Evaluation of Photocatalytical Efficacies of Pure and Composite Oxides
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barber, J. Photosynthetic Energy Conversion: Natural and Artificial. Chem. Soc. Rev. 2009, 38, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Available online: www.theworldcounts.com (accessed on 1 September 2022).
- Priya, A.K.; Suresh, R.; Kumar, P.S.; Rajendran, S.; Vo, D.-V.N.; Soto-Moscoso, M. A Review on Recent Advancements in Photocatalytic Remediation for Harmful Inorganic and Organic Gases. Chemosphere 2021, 284, 131344. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Xu, Y.; Akbari, M.Z.; Liang, C.; Peng, L. Insight into Integration of Photocatalytic and Microbial Wastewater Treatment Technologies for Recalcitrant Organic Pollutants: From Sequential to Simultaneous Reactions. Chemosphere 2022, 295, 133952. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Zhang, X.; Tryk, D. TiO2 Photocatalysis and Related Surface Phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Carević, M.; Abazović, N.D.; Savić, T.; Novaković, T.B.; Mojović, M.D.; Čomor, M.I. Structural, Optical and Photodegradation Properties of Pure and Fe-Doped Titania Nanoparticles Probed Using Simulated Solar Light. Ceram. Int. 2016, 42, 1521–1529. [Google Scholar] [CrossRef]
- Carević, M.V.; Abazović, N.D.; Novaković, T.B.; Pavlović, V.B.; Čomor, M.I. Zirconium Dioxide Nanopowders with Incorporated Si4+ Ions as Efficient Photocatalyst for Degradation of Trichlorophenol Using Simulated Solar Light. Appl. Catal. B-Environ. 2016, 195, 112–120. [Google Scholar] [CrossRef]
- Carević, M.V.; Savić, T.D.; Abazović, N.D.; Mojović, M.D.; Novaković, T.B.; Čomor, M.I. Effect of Fe3+ Ion Doping on Photocatalytic Ability of Nanozirconia Ceramic to Degrade 2,4,6-Trichlorophenol. Ceram. Int. 2020, 46, 6820–6827. [Google Scholar] [CrossRef]
- Abramović, B.; Šojić, D.; Anderluh, V.; Abazović, N.; Čomor, M. Nitrogen-Doped TiO2 Suspensions in Photocatalytic Degradation of Mecoprop and (4-Chloro-2-Methylphenoxy)Acetic Acid Herbicides Using Various Light Sources. Desalination 2009, 244, 293–302. [Google Scholar] [CrossRef]
- Abazović, N.D.; Montone, A.; Mirenghi, L.; Janković, I.A.; Čomor, M.I. TiO2 Doped with Nitrogen: Synthesis and Characterization. J. Nanosci. Nanotechno. 2008, 8, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Čomor, M.I.; Janković, I.A.; Savić, T.D.; Abazović, N.D.; Carević, M.V. Surface Modification of Anatase Nanoparticles with Catecholate- and Salicylate-type Ligands: A Novel Tool for Tuning Optical Properties. In Advances in Materials Science Research; Wythers, M.C., Ed.; Nova Science: Hauppauge, NY, USA, 2016; Volume 22, pp. 157–207. [Google Scholar]
- Carević, M.V.; Abazović, N.D.; Savić, T.D.; Novaković, T.B.; Pjević, D.J.; Čomor, M.I. Binary Oxide Ceramics for Enhanced Phenols Degradation under Simulated Solar Light. J. Am. Ceram. Soc. 2017, 101, 1420–1431. [Google Scholar] [CrossRef]
- Radoičić, M.; Šaponjić, Z.; Janković, I.A.; Ćirić-Marjanović, G.; Ahrenkiel, S.P.; Čomor, M.I. Improvements to the Photocatalytic Efficiency of Polyaniline Modified TiO2 Nanoparticles. Appl. Catal. B-Environ. 2013, 136–137, 133–139. [Google Scholar] [CrossRef]
- Hussain, I.; Jalil, A.A.; Hamid, M.Y.S.; Hassan, N.S. Recent Advances in Catalytic Systems in the Prism of Physicochemical Properties to Remediate Toxic CO Pollutants: A State-of-The-Art Review. Chemosphere 2021, 277, 130285. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, P.A.; Polisetti, S.; Madras, G. Rapid Synthesis of Ultrahigh Adsorption Capacity Zirconia by a Solution Combustion Technique. Langmuir 2011, 27, 3578–3587. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, X.; Yuan, L.; Yu, J. A Review of High-Temperature Electrochemical Sensors Based on Stabilized Zirconia. Solid State Ionics 2015, 283, 91–102. [Google Scholar] [CrossRef]
- Shim, J.H.; Chao, C.-C.; Huang, H.; Prinz, F.B. Atomic Layer Deposition of Yttria-Stabilized Zirconia for Solid Oxide Fuel Cells. Chem. Mater. 2007, 19, 3850–3854. [Google Scholar] [CrossRef]
- Basahel, S.N.; Ali, T.T.; Mokhtar, M.; Narasimharao, K. Influence of Crystal Structure of Nanosized ZrO2 on Photocatalytic Degradation of Methyl Orange. Nanoscale Res. Lett. 2015, 10, 73. [Google Scholar] [CrossRef] [Green Version]
- López, M.C.U.; Lemus, M.A.A.; Hidalgo, M.C.; González, R.L.; Owen, P.Q.; Oros-Ruiz, S.; López, S.A.U.; Acosta, J. Synthesis and Characterization of ZnO-ZrO2 Nanocomposites for Photocatalytic Degradation and Mineralization of Phenol. J. Nanomater. 2019, 2019, 1015876. [Google Scholar] [CrossRef] [Green Version]
- Ziółek, M.; Cohen, B.; Yang, X.; Sun, L.; Paulose, M.; Varghese, O.K.; Grimes, C.A.; Douhal, A. Femtosecond to Millisecond Studies of Electron Transfer Processes in a Donor–(π-Spacer)–Acceptor Series of Organic Dyes for Solar Cells Interacting with Titania Nanoparticles and Ordered Nanotube Array Films. Phys. Chem. Chem. Phys. 2012, 14, 2816. [Google Scholar] [CrossRef]
- Karunakaran, C.; Dhanalakshmi, R.; Gomathisankar, P. Photomineralization of Phenol on Al2O3: Synergistic Photocatalysis by Semiconductors. Res. Chem. Intermediat. 2010, 36, 361–371. [Google Scholar] [CrossRef]
- Tzompantzi, F.; Piña, Y.; Mantilla, A.; Aguilar-Martínez, O.; Galindo-Hernández, F.; Bokhimi, X.; Barrera, A. Hydroxylated Sol–Gel Al2O3 as Photocatalyst for the Degradation of Phenolic Compounds in Presence of UV Light. Catal. Today 2014, 220–222, 49–55. [Google Scholar] [CrossRef]
- Piña-Pérez, Y.; Tzompantzi-Morales, F.; Pérez-Hernández, R.; Arroyo-Murillo, R.; Acevedo-Peña, P.; Gómez-Romero, R. Photocatalytic Activity of Al2O3 Improved by the Addition of Ce3+/Ce4+ Synthesized by the Sol-Gel Method. Photodegradation of Phenolic Compounds Using UV Light. Fuel 2017, 198, 11–21. [Google Scholar] [CrossRef]
- Piña-Pérez, Y.; Aguilar-Martínez, O.; Oros-Ruíz, S.; Gómez, R.; Tzompantzi, F. Commercial Aluminum Oxides with Different Crystalline Structures Efficient for the Mineralization of Phenolic Pollutants. J. Photochem. Photobiol. A 2018, 353, 409–415. [Google Scholar] [CrossRef]
- Yoldas, B.E. Alumina Gels That Form Porous Transparent Al2O3. J. Mater. Sci. 1975, 10, 1856–1860. [Google Scholar] [CrossRef]
- Lippens, B. Studies on Pore Systems in Catalysts I. The Adsorption of Nitrogen; Apparatus and Calculation. J. Catal. 1964, 3, 32–37. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Cong, Y.; Dong, B.; Yu, N.; He, Y.; Zhao, Y.; Yang, Y. Enhanced Upconversion Emission in ZrO2–Al2O3 Composite Oxide. Dalton Trans. 2016, 45, 6627–6633. [Google Scholar] [CrossRef]
- Turakulova, A.O.; Golubina, E.V.; Lokteva, E.S.; Korotkov, A.V.; Lunin, V.V. ZrO2-Al2O3 Binary Oxides as Promising Supports for Palladium Catalysts of Hydrodechlorination. Russ. J. Phys. Chem. A 2011, 85, 402–407. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Ojha, A.K. Oxygen Vacancy Induced Photoluminescence Properties and Enhanced Photocatalytic Activity of Ferromagnetic ZrO2 Nanostructures on Methylene Blue Dye under Ultra-Violet Radiation. J. Alloy Compd. 2015, 644, 654–662. [Google Scholar] [CrossRef]
- Amirsalari, A.; Shayesteh, S.F. Effects of pH and calcination temperature on structural and optical properties of alumina nanoparticles. Superlattice Microst. 2015, 82, 507–524. [Google Scholar] [CrossRef]
- Guerrero-Araque, D.; Ramírez-Ortega, D.; Acevedo-Peña, P.; Tzompantzi, F.; Calderón, H.A.; Gómez, R. Interfacial Charge-Transfer Process across ZrO2-TiO2 Heterojunction and Its Impact on Photocatalytic Activity. J. Photochem. Photobiol. A 2017, 335, 276–286. [Google Scholar] [CrossRef] [Green Version]
- Bard, A.L.; Foulkner, L.R. Electrochemical Methods Fundamentals and Applications; John Wiley & Sons: New York, NY, USA, 2001. [Google Scholar]
- Ismael, M.; Wu, Y.; Wark, M. Photocatalytic Activity of ZrO2 Composites with Graphitic Carbon Nitride for Hydrogen Production under Visible Light. New J. Chem. 2019, 43, 4455–4462. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, X.; Li, G.; Cao, Y.; Shao, Y.; Li, D. Highly Efficient Bi2O2CO3/BiOCl Photocatalyst Based on Heterojunction with Enhanced Dye-Sensitization under Visible Light. Appl. Catal. B-Environ. 2016, 187, 301–309. [Google Scholar] [CrossRef]
- Kennedy, J.H.; Frese, K.W. Flatband Potentials and Donor Densities of Polycrystalline α - Fe2O3 Determined from Mott-Schottky Plots. J. Electrochem. Soc. 1978, 125, 723–726. [Google Scholar] [CrossRef]
- Dionysiou, D.D.; Khodadoust, A.P.; Kern, A.M.; Suidan, M.T.; Baudin, I.; Laîné, J.-M. Continuous-Mode Photocatalytic Degradation of Chlorinated Phenols and Pesticides in Water Using a Bench-Scale TiO2 Rotating Disk Reactor. Appl. Catal. B-Environ. 2000, 24, 139–155. [Google Scholar] [CrossRef]
- Karunakaran, C.; Dhanalakshmi, R. Semiconductor-Catalyzed Degradation of Phenols with Sunlight. Sol. Energy Mater. Sol. Cells 2008, 92, 1315–1321. [Google Scholar] [CrossRef]
- Hassan, N.S.; Jalil, A.A. A Review on Self-Modification of Zirconium Dioxide Nanocatalysts with Enhanced Visible-Light-Driven Photodegradation of Organic Pollutants. J. Hazard. Mater. 2022, 423, 126996. [Google Scholar] [CrossRef]






| Sample | SBET (m2g−1) | Vp0.98 (cm3g−1) | Vp micro (cm3g−1) | Dmax1 (nm) | Dmax2 (nm) | D (nm), from FWHM/XRD |
|---|---|---|---|---|---|---|
| Al2O3 | 280 | 0.441 | 0.104 | 4.9 | 3.1 | |
| 2 Al:1 Zr | 171 | 0.124 | 0.056 | 3.2 | 6.3 | NA |
| 1 Al:1 Zr | 145 | 0.154 | 0.044 | 3.2 | 6.4 | 5.7 |
| 1 Al:2 Zr | 90 | 0.167 | 0.037 | 3.8 | 7.2 | 7.5 |
| ZrO2 | 55 | 0.228 | 0.019 | 10.9 | 10–11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vulić, T.D.; Carević, M.V.; Abazović, N.D.; Novaković, T.B.; Mojović, Z.D.; Čomor, M.I. Application of Zirconia/Alumina Composite Oxide Ceramics as Photocatalysts for Removal of 2,4,6-Trichlorophenol from Water. Photochem 2022, 2, 905-917. https://doi.org/10.3390/photochem2040058
Vulić TD, Carević MV, Abazović ND, Novaković TB, Mojović ZD, Čomor MI. Application of Zirconia/Alumina Composite Oxide Ceramics as Photocatalysts for Removal of 2,4,6-Trichlorophenol from Water. Photochem. 2022; 2(4):905-917. https://doi.org/10.3390/photochem2040058
Chicago/Turabian StyleVulić, Tatjana D., Milica V. Carević, Nadica D. Abazović, Tatjana B. Novaković, Zorica D. Mojović, and Mirjana I. Čomor. 2022. "Application of Zirconia/Alumina Composite Oxide Ceramics as Photocatalysts for Removal of 2,4,6-Trichlorophenol from Water" Photochem 2, no. 4: 905-917. https://doi.org/10.3390/photochem2040058