Quaternization of Porous Cellulose Beads and Their Use for Removal of Humic Acid from Aqueous Medium
Abstract
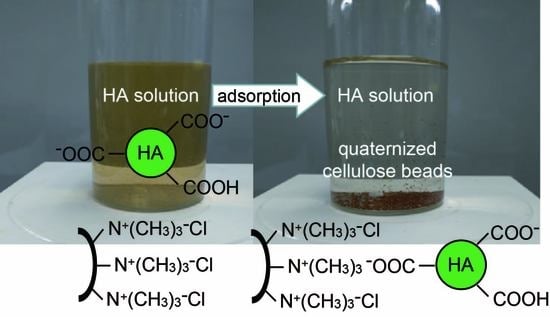
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Quaternization of Cellulose Beads
2.3. Determination of Content of Quaternary Ammonium Groups
2.4. FT-IR and XPS Analysis
2.5. Particle Size Distribution
2.6. Water Absorptivity
2.7. Preparation of HA Solutions
2.8. Adsorption of HA
2.9. Kinetic and Isothermal Analysis
2.10. Desorption
3. Results and Discussion
3.1. Preparation of Quaternized Cellulose
3.2. FT-IR and XPS Analysis
3.3. Water Absorptivity
3.4. HA Adsorption
3.4.1. The pH Dependence
3.4.2. The Temperature Dependence
3.4.3. The Content of Quaternary Ammonium Groups
3.4.4. The Dose of Quaternized Cellulose Beads
3.4.5. Kinetic Analysis
3.4.6. Analysis by Langmuir and Freundlich Adsorption Isotherms
3.5. Comparison with Other Adsorbents
3.6. Desorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klučáková, M.; Pelikán, P.; Lapčík, L.; Lapčiková, B.; Kučerík, J.; Kaláb, M. Structure and properties of humic and fulvic acids. 1. Properties and reactivity of humic acids and fulvic acids. J. Polym. Mater. 2000, 17, 337–356. [Google Scholar]
- Zhan, Y.; Zhu, Z.; Lin, J.; Qiu, Y.; Zhao, J. Removal of humic acid from aqueous solution by cetylpyridinium bromide modified zeolite. J. Environ. Sci. 2010, 22, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Anirudhan, T.S.; Suchithra, P.S.; Rijith, S. Amine-modified polyacrylamide-bentonite composite for the adsorption of humic acid in aqueous solutions. Colloids Surf. A Physicochem. Eng. Asp. 2008, 326, 147–156. [Google Scholar] [CrossRef]
- De Melo, B.A.G.; Motta, F.L.; Santana, M.H.A. Humic acids: Structural properties and multiple functionalities for novel technological developments. Mater. Sci. Eng. C. 2016, 62, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Murbach, T.S.; Glávits, R.; Endres, J.R.; Clewell, A.E.; Hirka, G.; Vértesi, A.; Szakonyiné, I.P. A toxicological evaluation of a fulvic and humic acids preparation. Toxicol. Rep. 2020, 7, 1242–1254. [Google Scholar] [CrossRef]
- Zhang, X.; Ding, Z.; Yang, J.; Cizmas, L.; Lichtfouse, E.; Sharma, V.K. Efficient microwave degradation of humic acids in water using persulfate and activated carbon. Environ. Chem. Lett. 2018, 16, 1069–1075. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Liu, J.; Li, L.; Zhen, G.; Lu, X.; Zhang, J.; Liu, H.; Zhou, Z.; Wu, Z.; Zhang, X. Prospects for humic acids treatment and recovery in wastewater: A review. Chemosphere 2023, 312, 137193. [Google Scholar] [CrossRef]
- Wall, N.A.; Choppin, G.R. Humic acids coagulation: Influence of divalent cations. Appl. Geochem. 2003, 18, 1573–1582. [Google Scholar] [CrossRef]
- Lowe, J.; Hossain, M.M. Application of ultrafiltration membranes for removal of humic acid from drinking water. Desalination 2008, 218, 343–354. [Google Scholar] [CrossRef]
- Doskoćil, L.; Grasset, L.; Válková, D.; Pakař, M. Hydrogen peroxide oxidation of humic acids and lignite. Fuel 2014, 134, 406–413. [Google Scholar] [CrossRef]
- Wen, P.; Liu, D.; Chen, W.; Jiang, G.; Li, Q. Decomposition of humic acid by ozone: Oxidation properties and water-matrix constituents. Desalination Water Treat. 2020, 174, 98–105. [Google Scholar] [CrossRef]
- Islam, M.A.; Morton, D.W.; Johnson, B.B.; Angove, M. Adsorption of humic and fulvic acids onto a range of adsorbents in aqueous systems, and their effect on the adsorption of other species: A review. Sep. Purif. Technol. 2020, 247, 116949. [Google Scholar] [CrossRef]
- Kołodziej, A.; Fuentes, M.; Baigorri, R.; Lorenc-Brabowska, E.; García-Mina, J.M.; Burg, P.; Gryglewicz, G. Mechanism of adsorption of different humic acid fractions on mesoporous activated carbons with basic surface characteristics. Adsorption 2014, 20, 667–675. [Google Scholar] [CrossRef]
- Eustáquio, H.M.B.; Lopes, C.W.; da Rocha, R.S.; Cardoso, B.D.; Pergher, S.B.C. Modification of activated carbon for the adsorption of humic acid. Adsorpt. Sci. Technol. 2015, 33, 117–126. [Google Scholar] [CrossRef]
- Habuta-stanić, M.; Tutić, A.; Grgić, D.K.; Zeko-Pivač, A.; Burilo, A.; Paixão, S.; Teixeira, V.; Pagaimo, M.; Pala, A.; Ravančić, M.E.; et al. Adsorption of humic acid from water using chemically modified bituminous coal-based activated carbons. Chem. Biochem. Eng. Q. 2021, 35, 189–203. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Hokkanen, S.; Bhatnagar, A.; Sillanpää, M. A review on modification methods to cellulose-based adsorbents to improve adsorption capacity. Water Res. 2016, 91, 156–173. [Google Scholar] [CrossRef]
- Wohlert, M.; Benselfelt, T.; Wågberg, L.; Furó, I.; Berglund, L.A.; Wohlert, J. Cellulose and the role of hydrogen bonds: Not in charge of everything. Cellulose 2022, 29, 1–23. [Google Scholar] [CrossRef]
- Peng, B.; Yao, Z.; Wang, X.; Crombeen, M.; Sweeney, D.G.; Tam, K.C. Cellulose-based materials in wastewater treatment of petroleum industry. Green Energy Environ. 2020, 5, 37–49. [Google Scholar] [CrossRef]
- Rana, A.K.; Mishra, Y.K.; Gupta, V.K.; Thakur, V.K. Sustainable materials in the removal of pesticides from contaminated water: Perspective on macro to nanoscale cellulose. Sci. Total Environ. 2021, 797, 149129. [Google Scholar] [CrossRef]
- Kaur, J.; Sengupta, P.; Mukhopadhyay, S. Critical review of bioadsorption on modified cellulose and removal of divalent heavy metals (Cd, Pb, and Cu). Ind. Eng. Chem. Res. 2022, 61, 1921–1954. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Mathew, A.P. Cellulose-based materials for water remediation: Adsorption, catalysis, and antifouling. Front. Chem. Eng. 2021, 3, 790314. [Google Scholar] [CrossRef]
- Kopač, T.; Krajnc, M.; Ručigaj, A. A rheological study of cationic micro- and nanofibrillated cellulose: Quaternization reaction optimization and fibril characteristic effects. Cellulose 2022, 29, 1435–1450. [Google Scholar] [CrossRef]
- Sehaqui, H.; de Larraya, U.P.; Tingaut, P.; Zimmermann, T. Humic acid adsorption onto cationic cellulose nanofibers for bioinspired removal of copper (II) and a positively charged dye. Soft Matter 2015, 11, 5294–5300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sehaqui, H.; Mautner, A.; de Larraya, U.P.; Pfenninger, N.; Tingaut, P.; Zimmermann, T. Cationic cellulose nanofibers from waste pulp residues and their nitrate, fluoride, sulphate and phosphate adsorption properties. Carbohyd. Polym. 2016, 135, 334–340. [Google Scholar] [CrossRef]
- Nishino, A.; Taki, A.; Asamoto, H.; Minamisawa, H.; Yamada, K. Kinetic, isotherm, and equilibrium investigation of Cr (VI) ion adsorption on amine-functionalized porous silica beads. Polymers 2022, 14, 2104. [Google Scholar] [CrossRef]
- Saha, B.; Orvig, C. Biosorbents for hexavalent chromium elimination from industrial and municipal effluents. Coord. Chem. Rev. 2010, 254, 2959–2972. [Google Scholar] [CrossRef]
- Parlayici, S.; Pehlivan, E. Comparative study of Cr(VI) removal by bio-waste adsorbents: Equilibrium, kinetics, and thermodynamic. J. Anal. Sci. Technol. 2019, 10, 15. [Google Scholar] [CrossRef] [Green Version]
- Ho, Y. Second-order kinetic model for the sorption of cadmium onto tree fern: A comparison of linear and non-linear methods. Water Res. 2006, 40, 119–125. [Google Scholar] [CrossRef]
- Ho, Y. Review of second-order models for adsorption systems. J. Hazard. Mater. 2006, B136, 681–689. [Google Scholar] [CrossRef] [Green Version]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H. Über die Adsorption in Lösungen. Zeit. Phys. Chem. 1907, 57, 385–470. [Google Scholar] [CrossRef]
- Wang, J.; Bi, L.; Ji, Y.; Ma, H.; Yin, X. Removal of humic acid from aqueous solution by magnetically separable polyaniline: Adsorption behavior and mechanism. J. Colloid Interface Sci. 2014, 430, 140–146. [Google Scholar] [CrossRef]
- Courtenay, J.C.; Hohns, M.A.; Galembeck, F.; Deneke, C.; Lanzoni, E.M.; Costa, C.A.; Scott, J.L.; Sharma, R.I. Surface modified cellulose scaffolds for tissue engineering. Cellulose 2017, 24, 253–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Udoetok, I.A.; Wilson, L.D.; Headley, J.V. Quaternized cellulose hydrogels as sorbent materials and pickering emulsion stabilizing agents. Materials 2016, 9, 645. [Google Scholar] [CrossRef] [Green Version]
- Quinlan, P.J.; Tanvir, A.; Tam, K.C. Application of the central composite design to study the flocculation of an anionic azo dye using quaternized cellulose nanofibrils. Carbohydr. Polym. 2015, 133, 80–89. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, H.; Wang, B.; Shi, S.; Xiong, L.; Chen, X. Synthesis and characterization of quaternized bacterial cellulose prepared in homogeneous aqueous solution. Carbohydr. Polym. 2016, 136, 171–176. [Google Scholar] [CrossRef]
- Song, Y.; Sun, Y.; Zhang, X.; Zhou, J.; Zhang, L. Homogeneous quaternization of cellulose in NaOH/urea aqueous solutions as gene carriers. Biomacromolecules 2008, 9, 2259–2264. [Google Scholar] [CrossRef]
- Yamada, K.; Kitao, K.; Asamoto, H.; Minamisawa, H. Development of recoverable adsorbents for Cr(VI) ions by grafting of a dimethylamino group-containing monomer on polyethylene substrate and subsequent quaternization. Environ. Technol. 2021. [Google Scholar] [CrossRef]
- Deng, S.; Bai, R. Adsorption and desorption of humic acid on aminated polyacrylonitrile fibers. J. Colloid Interface Sci. 2004, 280, 36–43. [Google Scholar] [CrossRef]
- Deng, S.; Yu, G.; Ting, Y. Removal of humic acid using PEI-modified fungal biomass. Separat. Sci. Technol. 2006, 41, 2989–3002. [Google Scholar] [CrossRef]
- Manirethan, V.; Raval, K.; Rajan, R.; Thaira, H.; Balakrishnan, R.M. Kinetic and thermodynamic studies on the adsorption of heavy metals from aqueous solution by melanin nanopigment obtained from marine source: Pseudomonas stutzeri. J. Environ. Manag. 2018, 214, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Rupiasih, N.N.; Vidyasagar, P.B. A review: Compositions, structures, properties and applications of humic substances. J. Adv. Sci. Technol. 2005, 8, 16–25. [Google Scholar]
- Sánchez-Monedero, M.A.; Cegarra, J.; García, D.; Roig, A. Chemical and structural evolution of humic acids during organic waste composting. Biodegradation 2002, 13, 361–371. [Google Scholar] [CrossRef]
- Avena, M.J.; Koopal, L.K. Kinetics of humic acid adsorption at solid-water interfaces. Environ. Sci. Technol. 1999, 33, 2739–2744. [Google Scholar] [CrossRef]
- Bousba, S.; Bougdah, N.; Messikh, N.; Magri, P. Adsorption removal of humic acid from water using a modified Algerian bentonite. Phys. Chem. Res. 2018, 6, 613–625. [Google Scholar]
- Zularisam, A.W.; Ismail, A.F.; Salim, R. Behaviours of natural organic matter in membrane filtration for surface water treatment—A review. Desalination 2006, 194, 211–231. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Ning, F.; Kang, M.; Ma, C.; Qiu, Z. Effective removal of humic acid from aqueous solution using adsorbents prepared from the modified waste bamboo powder. Microchem. J. 2020, 153, 104272. [Google Scholar] [CrossRef]
- Taktak, F.; Ìbay, Z. Synthesis of novel poly[2-(dimethylamino) ethyl methacrylate]/pumice stone hydrogel composite for the rapid adsorption of humic acid in aqueous solution. J. Macromol. Sci. A Pure Appl. Chem. 2015, 52, 307–315. [Google Scholar] [CrossRef]
- Chen, K.; Feng, Q.; Feng, Y.; Ma, D.; Wang, D.; Liu, Z.; Zhu, W.; Li, X.; Qin, F.; Feng, J. Ultrafast removal of humic acid by amine-modified silica aerogel: Insights from experiments and density functional theory calculation. Chem. Eng. J. 2022, 435, 135171. [Google Scholar] [CrossRef]
- Zhang, J.; Kang, M.; Zhou, Y.; Ma, C.; Ning, F.; Qiu, Z. Facile synthesis of polyethyleneimine modified magnetic graphite: An effective adsorbent for the removal of humic acid from aqueous solution. Mater. Chem. Phys. 2020, 255, 123549. [Google Scholar] [CrossRef]
- Du, J.; Dong, Z.; Pi, Y.; Yang, X.; Zhao, L. Fabrication of cotton linter-based adsorbents by radiation grafting polymerization for humic acid removal from aqueous solution. Polymers 2019, 11, 962. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, S. Removal of humic acid from aqueous solution using polyacrylamide/chitosan semi-IPN hydrogel. Water Sci. Technol. 2018, 2017, 16–26. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y. Electrospun cellulose-acetate/chitosan fibers for humic-acid removal: Improved efficiency and robustness with a core-sheath design. Namomaterials 2022, 12, 1284. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, F.; Yixiang, W. Electrospun cellulose acetate/chitosan fibers for humic acid removal: Construction guided by intermolecular interaction study. Appl. Polym. Mater. 2021, 3, 5022–5029. [Google Scholar] [CrossRef]












| Content of Quaternary Ammonium Groups (mmol/g) | Untreated | 0.053 | 0.231 | 0.380 | 0.524 |
|---|---|---|---|---|---|
| in the dry state | |||||
| Average size (mm) | 0.298 | 0.299 | 0.293 | 0.302 | 0.294 |
| Standard deviation (mm) | 0.046 | 0.050 | 0.048 | 0.041 | 0.038 |
| in the water swollen state | |||||
| Average size (mm) | 0.395 | 0.394 | 0.393 | 0.405 | 0.399 |
| Standard deviation (mm) | 0.048 | 0.049 | 0.050 | 0.045 | 0.037 |
| pH | Qexp | Qcal | Relative Error | k1 | r2 | Time Range |
| (g/g) | (g/g) | (%) | (1/h) | (−) | (min) | |
| 3 | 0.359 | 0.35 | −2.36 | 1.667 | 0.9088 | 5 |
| 6 | 0.195 | 0.192 | −1.49 | 0.504 | 0.9952 | 40 |
| 9 | 0.195 | 0.187 | −4.09 | 1.253 | 0.9938 | 5 |
| pH | Qexp | Qcal | Relative Error | k2 | r2 | Time Range |
| (g/g) | (g/g) | (%) | (g/g·h) | (−) | (h) | |
| 3 | 0.359 | 0.355 | −1.14 | 2.288 | 0.9988 | 7 |
| 6 | 0.195 | 0.194 | −0.55 | 3.072 | 0.9996 | 8 |
| 9 | 0.195 | 0.199 | 1.99 | 2.943 | 0.995 | 7 |
| Langmuir Isotherm | Freundlich Isotherm | ||||
|---|---|---|---|---|---|
| pH 3.0 | pH 6.0 | pH 3.0 | pH 6.0 | ||
| Qmax (g/g) | 0.775 | 0.378 | n | 1.86 | 2.17 |
| KL (dm3/mg) | 0.0313 | 0.0174 | KF (mg/g)/(dm3/mg)1/n | 0.0548 | 0.0288 |
| r2 | 0.9895 | 0.9996 | r2 | 0.9866 | 0.9925 |
| Adsorbent | pH | Temp. | HA Conc. | Dose | Q | Ref. |
|---|---|---|---|---|---|---|
| (°C) | (mg/L) | (g/L) | (mg/g) | |||
| quaternized cellulose beads | 3.0 | 25 | 100 | 0.20 | 359 | this study |
| cetylpyridium bromide modified zeolite | 7.5 | 30 | 60 | 0.40 | 92 | [2] |
| amine-modified PAAM-bentonite composite | 6 | 50 | 167.5 | 2.00 | 82.6 | [3] |
| quaternized cellulose fibers | 6 | room temp. | 500 | 0.50 | 310 | [24] |
| magnetic Fe3O4@SiO2-PANI particle | 6 | 25 | 41.85 | 0.50 | 36.36 | [26] |
| PEI-modified fungal biomass | 5 | 25 | 100 | 1.00 | 41.5 | [42] |
| Fe3O4@Na-BP-PEI particles | 6.7 | 20 | 91 | 0.20 | 114 | [49] |
| PDMAEMA/pumice stone hydrogel composite | 4 | 20 | 50 | 0.50 | 59.72 | [50] |
| amine-modified silica aerogel | 5 | 35 | 250 | 1.00 | 236.4 | [51] |
| PEI modified magnetic graphite | 4 | 20 | 21 | 0.25 | 83.71 | [52] |
| quaternized DMAEMA grafted cotton linter | 6 | 30 | 800 | 1.00 | 333.3 | [53] |
| PAAm/chitosan semi IPN hydrogel | 7 | 25 | 80 | 0.50 | 148.7 | [54] |
| chitosan/cellulose acetate-cellulose nanocrystal fibers | 4.0 | 25 | 30 | 0.06 | 151.4 | [55] |
| electrospun cellulose acetate/chitosan fibers | 4.0 | 25 | 30 | 0.04 | 184.7 | [56] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uchiyama, K.; Asamoto, H.; Minamisawa, H.; Yamada, K. Quaternization of Porous Cellulose Beads and Their Use for Removal of Humic Acid from Aqueous Medium. Physchem 2023, 3, 61-76. https://doi.org/10.3390/physchem3010005
Uchiyama K, Asamoto H, Minamisawa H, Yamada K. Quaternization of Porous Cellulose Beads and Their Use for Removal of Humic Acid from Aqueous Medium. Physchem. 2023; 3(1):61-76. https://doi.org/10.3390/physchem3010005
Chicago/Turabian StyleUchiyama, Kana, Hiromichi Asamoto, Hiroaki Minamisawa, and Kazunori Yamada. 2023. "Quaternization of Porous Cellulose Beads and Their Use for Removal of Humic Acid from Aqueous Medium" Physchem 3, no. 1: 61-76. https://doi.org/10.3390/physchem3010005