Density-Driven Convection for CO2 Solubility Trapping in Saline Aquifers: Modeling and Influencing Factors
Abstract
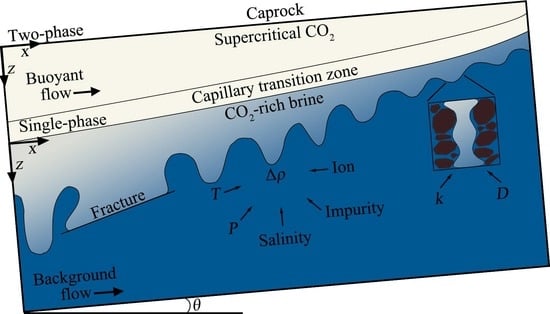
:1. Introduction
2. Modeling
2.1. Modeling Methodology
2.2. Coordinate Selection and Boundary Conditions
3. Influencing Factors
3.1. Fluid Dynamics
3.1.1. Permeability
3.1.2. Porosity
3.1.3. Fractures and Stratification
3.1.4. Slope of Caprock
3.1.5. Hydrodynamic Dispersion
3.2. Dissolution Dynamics
3.2.1. Temperature, Pressure, and Salinity
3.2.2. Ions and Impurities
4. Conclusions and Future Prospects
- (1)
- The simplification of the single-phase system in a two-dimensional ideal rectangular porous medium will lead to an incorrect estimate of the CO2 convective onset time and flux. The two-phase system additionally considers the capillary transition zone to correct this misestimate. Density change is the main key to density-driven convection and is controlled linearly by CO2 concentration. The geothermal gradient prevalent in the saline aquifer will also trigger a density change, which is described by introducing an energy equation into modeling. However, the effect of this additional convection will not be significant. The viscosity difference of fluids can trigger similar transversal convection early in CO2 injection and should be discussed in the context of studies related to structural trapping, as its role is insignificant compared to density difference after CO2 dissolution has generally occurred. The background flow can be introduced by a fixed velocity boundary condition that would significantly delay the onset of density-driven convection. To fully approximate the real CO2 saline aquifer sequestration situation, future studies should consider and model the modeling simplifications comprehensively and appropriately.
- (2)
- Permeability is an important property of aquifers and determines the magnitude of Ra that controls convection. An increase in permeability advances the onset of convection, which often implies a larger CO2 dissolution flux with a larger number and wavelength of convective fingers. This leads to more CO2 dissolution and is positive for CO2 solubility trapping in saline aquifers. The heterogeneity of the saline aquifer is taken into account through several permeability representations, and similar to the findings in homogeneous porous media, fluctuating and enhancing permeability in either direction in heterogeneous saline aquifers promotes CO2 density-driven convection. It is important to note that incomplete consideration of the sensitivity analysis may produce a biased perception of the permeability anisotropy impact on CO2 density-driven convection. This requires further research to gain insight into the heterogeneity of saline aquifers since it is frequently observed in CO2 sequestration projects.
- (3)
- The porosity significantly influences the density-driven convection by controlling the pattern of CO2-rich brine front in saline aquifers. For low porosity, CO2-rich brine maintained the form of a stable boundary layer that slowly diffused downward. Furthermore, the convective fingering is clearly observed in the saline aquifer with high porosity. Implying a thinner diffusion boundary layer, an earlier onset of convection, and a smaller Sh. This is explained as an effect caused by the change in the effective diffusion coefficient. Meanwhile, the effect caused by varying the permeability is insignificant. The porosity heterogeneity is introduced into the numerical model as fluctuation, leading to instability and correlating with convection onset time. As the porosity fluctuation increases, the convection onset time decreases and is more significant than the permeability fluctuation. An optimal porosity fluctuation that minimizes the convection onset time exists. This suggests that even small porosity fluctuations are sufficient to trigger nonlinear convection. Future research and CO2 sequestration projects should take this factor fully into account.
- (4)
- In a single fracture system, the greater inclination angle of the fracture is equivalent to providing a preferential channel for solute transport, which facilitates the solubility trapping process. In the more complex multi-fracture systems, the situation can be more complicated. In general, regarding the concerned results, the impact of fractures on convection should be further emphasized, which will be more relevant to the practical applications of CO2 solubility trapping. In stratified heterogeneous formations, the arrangement of layers with different permeability for convection has been extensively studied, and although there are relevant findings at each scale, from pore to the field, the corresponding up/downscale studies are still relatively rare, but they are overwhelmingly important for the practical application of CO2 sequestration. Different lithologies are associated with the stratification of aquifers, and this affects the structural geometry and aquifer properties. The quantity and relative spacing of internal laminae or boundary surfaces that are horizontally or obliquely oriented, which impede fluid flow, account for a major portion of permeability variances in facies. The Permian White Rim Sandstone is the best reservoir for CO2 injection, with great permeability and porosity. The composition of the rocks, sedimentary diagnostic eolian features, thickness and depth of the layers, etc., should all be considered to judge the applicability of geological storage. Transmissibility plays an important role in the stratification and subsequent fault of aquifers. A saline aquifer with optimal transmissibility may not be the best option for CO2 sequestration safety. Other risks in CO2 sequestration projects still need to be assessed to find the best solution.
- (5)
- The slope of caprock is prevalent in saline aquifers and is usually considered to be the top boundary tilt in the model. The slope of caprock will induce massive transversal movement, merging, and coarsening of convective fingering, which implies an enhancement of density-driven convection. However, the increasing slope will lead to an excessive migration of CO2, which increases the risk of contamination from CO2 exposure to freshwater. More reservoir parameters and chemical reactions associated with the caprock should be considered in further studies to achieve a more realistic calculation of optimal slope that can guide the site selection for CO2 sequestration projects.
- (6)
- Some contradictory conclusions are reached on the effect of hydraulic dispersion, and the deviations may stem from the non-independence of the dimensionless number and can be resolved by specific dimensionless methods. It shows that an increase in the dispersion ratio accelerates the onset of density-driven convection. Nevertheless, it is important to further explore the practical effects of hydraulic dispersion anisotropy in conjunction with experiments to gain insight and a consistent conclusion. Density-driven convection becomes more complex when both background flow and diffusion are considered, and further research is needed to provide as much guidance as possible for CO2 sequestration projects.
- (7)
- The increase in pressure and decrease in temperature or salinity in the aquifer will promote the dissolution of CO2 in brine, which accelerates the development of density-driven convection. It is important to note that the positive impact of increased pressure on CO2 solubility trapping comes with a burden on caprock, particularly in a naturally fractured one. The ability of saline aquifers to sequester CO2 is limited by this risk, and future studies should consider the interaction between these parameters to maximize CO2 sequestration with acceptable risk. The brine properties also significantly influence the dissolution of CO2. A low pH and high concentration of divalent ions in brine will inhibit the dissolution of CO2, and, conversely, an aquifer composed of carbonate rocks will accelerate this process. Moreover, for reasons of economic efficiency and environmental protection, CO2 injection into the saline aquifer is frequently accompanied by impurities. It is generally accepted that SO2 has a greater solubility density, and this will enhance density-driven convection, while N2 and H2S show the opposite impact. However, the actual effect of impurities on density-driven convection may be non-monotonic, depending on the molar percentage of impurities in brine. Chromatographic partitioning should also be noted, as it accelerates density-driven convection in some cases. For reasons of economy and safety, a more specific study of these effects is awaited, as there is an optimum impurity ratio to enhance density-driven convection. These saline aquifer parameters also significantly affect CO2 structural and mineral trapping, thus revealing that the actual saline aquifer sequestration of CO2 is complex and involves multiple trapping mechanisms. For an accurate assessment of the ability of saline aquifers to sequester CO2, other trapping mechanisms and their coupling with solubility trapping should be taken into account.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shafabakhsh, P.; Ataie-Ashtiani, B.; Simmons, C.T.; Younes, A.; Fahs, M. Convective-reactive transport of dissolved CO2 in fractured-geological formations. Int. J. Greenh. Gas Control 2021, 109, 103365. [Google Scholar] [CrossRef]
- Rawshan Ara Begum, R.; Lempert, R.; Ali, T.A.B.E.; Bernauer, T.; Cramer, W.; Cui, X.; Mach, K.; Nagy, G.; Stenseth, N.C.; Sukumar, R.; et al. Point of Departure and Key Concepts. In Climate Change 2022: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Pörtner, H.O., Roberts, D.C., Tignor, M., Poloczanska, E.S., Mintenbeck, K., Alegría, A., Craig, M., Langsdorf, S., Löschke, S., Möller, V., et al., Eds.; Cambridge University Press: Cambridge, UK/ New York, NY, USA, 2022; pp. 121–196. [Google Scholar]
- Young-Lorenz, J.D.; Lumley, D. Portfolio Analysis of Carbon Sequestration Technologies and Barriers to Adoption: General Methodology and Application to Geological Storage. Energy Procedia 2013, 37, 5063–5079. [Google Scholar] [CrossRef] [Green Version]
- Dai, Z.; Viswanathan, H.; Middleton, R.; Pan, F.; Ampomah, W.; Yang, C.; Jia, W.; Xiao, T.; Lee, S.-Y.; McPherson, B.; et al. CO2 Accounting and Risk Analysis for CO2 Sequestration at Enhanced Oil Recovery Sites. Environ. Sci. Technol. 2016, 50, 7546–7554. [Google Scholar] [CrossRef]
- Aminu, M.D.; Nabavi, S.A.; Rochelle, C.A.; Manovic, V. A review of developments in carbon dioxide storage. Appl. Energy 2017, 208, 1389–1419. [Google Scholar] [CrossRef] [Green Version]
- Shukla, R.; Ranjith, P.; Haque, A.; Choi, X. A review of studies on CO2 sequestration and caprock integrity. Fuel 2010, 89, 2651–2664. [Google Scholar] [CrossRef]
- Jiang, X. A review of physical modelling and numerical simulation of long-term geological storage of CO2. Appl. Energy 2011, 88, 3557–3566. [Google Scholar] [CrossRef]
- Emami-Meybodi, H.; Hassanzadeh, H.; Green, C.P.; Ennis-King, J. Convective dissolution of CO2 in saline aquifers: Progress in modeling and experiments. Int. J. Greenh. Gas Control 2015, 40, 238–266. [Google Scholar] [CrossRef]
- Alcalde, J.; Flude, S.; Wilkinson, M.; Johnson, G.; Edlmann, K.; Bond, C.E.; Scott, V.; Gilfillan, S.M.V.; Ogaya, X.; Haszeldine, R.S. Estimating geological CO2 storage security to deliver on climate mitigation. Nat. Commun. 2018, 9, 2201. [Google Scholar] [CrossRef]
- Dai, Z.; Middleton, R.; Viswanathan, H.; Fessenden-Rahn, J.; Bauman, J.; Pawar, R.; Lee, S.-Y.; McPherson, B. An Integrated Framework for Optimizing CO2 Sequestration and Enhanced Oil Recovery. Environ. Sci. Technol. Lett. 2014, 1, 49–54. [Google Scholar] [CrossRef]
- Dance, T.; Paterson, L. Observations of carbon dioxide saturation distribution and residual trapping using core analysis and repeat pulsed-neutron logging at the CO2CRC Otway site. Int. J. Greenh. Gas Control 2016, 47, 210–220. [Google Scholar] [CrossRef]
- Ritzi, R.W.; Freiburg, J.T.; Webb, N.D. Understanding the (co)variance in petrophysical properties of CO2 reservoirs comprising sedimentary architecture. Int. J. Greenh. Gas Control 2016, 51, 423–434. [Google Scholar] [CrossRef] [Green Version]
- Freiburg, J.T.; Morse, D.G.; Leetaru, H.E.; Hoss, R.P.; Yan, Q. A Depositional and Diagenetic Characterization of the Mt. Simon Sandstone at the Illinois Basin—Decatur Project Carbon Capture and Storage Site, Decatur, Illinois, USA; Illinois State Geological Survey, Prairie Research Institute, University of Illinois: Champaign, IL, USA, 2014. [Google Scholar]
- Tye, R.S.; Watson, B.A.; McGuire, P.L.; Maguire, M.M.; Carr, T.R.; Mason, E.P.; Feazel, C.T. Unique Horizontal-well Designs Boost Primary and EOR Production, Prudhoe Bay Field, Alaska. In Horizontal Wells: Focus on the Reservoir; American Association of Petroleum Geologists: Tulsa, OK, USA, 2003; Volume 14, pp. 113–125. [Google Scholar]
- Flett, M.; Gurton, R.; Weir, G. Heterogeneous saline formations for carbon dioxide disposal: Impact of varying heterogeneity on containment and trapping. J. Pet. Sci. Eng. 2007, 57, 106–118. [Google Scholar] [CrossRef]
- Ershadnia, R.; Wallace, C.; Soltanian, R. CO2 geological sequestration in heterogeneous binary media: Effects of geological and operational conditions. Adv. Geo-Energy Res. 2020, 4, 392–405. [Google Scholar] [CrossRef]
- Wang, Y.; Vuik, C.; Hajibeygi, H. CO2 Storage in deep saline aquifers: Impacts of fractures on hydrodynamic trapping. Int. J. Greenh. Gas Control 2022, 113, 103552. [Google Scholar] [CrossRef]
- Mahyapour, R.; Mahmoodpour, S.; Singh, M.; Omrani, S. Effect of permeability heterogeneity on the dissolution process during carbon dioxide sequestration in saline aquifers: Two-and three-dimensional structures. Geomech. Geophys. Geo-Energy Geo-Resour. 2022, 8, 70. [Google Scholar] [CrossRef]
- Rezk, M.G.; Foroozesh, J. Study of convective-diffusive flow during CO2 sequestration in fractured heterogeneous saline aquifers. J. Nat. Gas Sci. Eng. 2019, 69, 102926. [Google Scholar] [CrossRef]
- Hewitt, D.R.; Neufeld, J.A.; Lister, J.R. Convective shutdown in a porous medium at high Rayleigh number. J. Fluid Mech. 2013, 719, 551–586. [Google Scholar] [CrossRef] [Green Version]
- Ren, F.; Ma, G.; Wang, Y.; Fan, L.; Zhu, H. Two-phase flow pipe network method for simulation of CO2 sequestration in fractured saline aquifers. Int. J. Rock Mech. Min. Sci. 2017, 98, 39–53. [Google Scholar] [CrossRef]
- Ahmed, R.; Li, J. A numerical framework for two-phase flow of CO2 injection into fractured water-saturated reservoirs. Adv. Water Resour. 2019, 130, 283–299. [Google Scholar] [CrossRef]
- Singh, M.; Chaudhuri, A.; Soltanian, M.R.; Stauffer, P.H. Coupled multiphase flow and transport simulation to model CO2 dissolution and local capillary trapping in permeability and capillary heterogeneous reservoir. Int. J. Greenh. Gas Control 2021, 108, 103329. [Google Scholar] [CrossRef]
- Kou, Z.; Wang, H.; Alvarado, V.; Fred McLaughlin, J.; Quillinan, S.A. Impact of sub-core scale heterogeneity on CO2/brine multiphase flow for geological carbon storage in the upper Minnelusa sandstones. J. Hydrol. 2021, 599, 126481. [Google Scholar] [CrossRef]
- Meng, Q.; Jiang, X. Numerical analyses of the solubility trapping of CO2 storage in geological formations. Appl. Energy 2014, 130, 581–591. [Google Scholar] [CrossRef]
- Luther, E.E.; Dallaston, M.C.; Shariatipour, S.M.; Holtzman, R. Onset of convective instability in an inclined porous medium. Phys. Fluids 2022, 34, 014104. [Google Scholar] [CrossRef]
- Zhang, C.; Kaito, K.; Hu, Y.; Patmonoaji, A.; Matsushita, S.; Suekane, T. Influence of stagnant zones on solute transport in heterogeneous porous media at the pore scale. Phys. Fluids 2021, 33, 036605. [Google Scholar] [CrossRef]
- Hewitt, D.R. Evolution of convection in a layered porous medium. J. Fluid Mech. 2022, 941, A56. [Google Scholar] [CrossRef]
- Ahkami, M.; Parmigiani, A.; Di Palma, P.R.; Saar, M.O.; Kong, X.-Z. A lattice-Boltzmann study of permeability-porosity relationships and mineral precipitation patterns in fractured porous media. Comput. Geosci. 2020, 24, 1865–1882. [Google Scholar] [CrossRef]
- Sohal, M.A.; Le Gallo, Y.; Audigane, P.; de Dios, J.C.; Rigby, S.P. Effect of geological heterogeneities on reservoir storage capacity and migration of CO2 plume in a deep saline fractured carbonate aquifer. Int. J. Greenh. Gas Control 2021, 108, 103306. [Google Scholar] [CrossRef]
- Li, D.; Zhang, H.; Li, Y.; Xu, W.; Jiang, X. Effects of N2 and H2S binary impurities on CO2 geological storage in stratified formation—A sensitivity study. Appl. Energy 2018, 229, 482–492. [Google Scholar] [CrossRef]
- Li, D.; Zhong, Y.; Jiang, X. Experimental study of impurity effects on convective mixing in Hele-Shaw cell with application to CO2 geological sequestration. Adv. Water Resour. 2023, 172, 104379. [Google Scholar] [CrossRef]
- Pau, G.S.H.; Bell, J.B.; Pruess, K.; Almgren, A.S.; Lijewski, M.J.; Zhang, K. High-resolution simulation and characterization of density-driven flow in CO2 storage in saline aquifers. Adv. Water Resour. 2010, 33, 443–455. [Google Scholar] [CrossRef]
- Yuan, H.-Z.; Zhang, X.-R. Numerical simulation with adaptive finite element methods for CO2 storage in saline aquifers. Int. Commun. Heat Mass Transf. 2013, 45, 55–63. [Google Scholar] [CrossRef]
- Amooie, M.A.; Soltanian, M.R.; Moortgat, J. Solutal convection in porous media: Comparison between boundary conditions of constant concentration and constant flux. Phys. Rev. E 2018, 98, 033118. [Google Scholar] [CrossRef] [Green Version]
- Bear, J. Dynamics of Fluids in Porous Media; American Elsevier Publishing Company, Inc.: New York, NY, USA, 1972. [Google Scholar]
- Whitaker, S. Flow in porous media I: A theoretical derivation of Darcy’s law. Transp. Porous Media 1986, 1, 3–25. [Google Scholar] [CrossRef]
- Lindeberg, E.; Wessel-Berg, D. Vertical convection in an aquifer column under a gas cap of CO2. Energy Convers. Manag. 1997, 38, S229–S234. [Google Scholar] [CrossRef]
- Javaheri, M.; Abedi, J.; Hassanzadeh, H. Linear Stability Analysis of Double-Diffusive Convection in Porous Media, with Application to Geological Storage of CO2. Transp. Porous Media 2010, 84, 441–456. [Google Scholar] [CrossRef]
- Oldenburg, C.M.; Rinaldi, A.P. Buoyancy Effects on Upward Brine Displacement Caused by CO2 Injection. Transp. Porous Media 2011, 87, 525–540. [Google Scholar] [CrossRef] [Green Version]
- Rasmusson, M.; Fagerlund, F.; Tsang, Y.; Rasmusson, K.; Niemi, A. Prerequisites for density-driven instabilities and convective mixing under broad geological CO2 storage conditions. Adv. Water Resour. 2015, 84, 136–151. [Google Scholar] [CrossRef]
- Sun, J.-J.; Zhu, Q.-Y.; Yu, H.-Z. The Influence of Temperature Gradient on the Convective Mixing Phenomena of Geologic Sequestration of CO2. Procedia Eng. 2015, 126, 421–425. [Google Scholar] [CrossRef] [Green Version]
- Gautam, K.; Narayana, P.A.L.; Hill, A.A. Thermo-convective carbon sequestration in horizontal porous layers. IMA J. Appl. Math. 2019, 84, 650–668. [Google Scholar] [CrossRef]
- Nield, D.A.; Bejan, A. Convection in Porous Media; Springer: Berlin, Germany, 2006; Volume 3. [Google Scholar]
- Diersch, H.J.G.; Kolditz, O. Variable-density flow and transport in porous media: Approaches and challenges. Adv. Water Resour. 2002, 25, 899–944. [Google Scholar] [CrossRef]
- Rogerson, A.; Meiburg, E. Shear stabilization of miscible displacement processes in porous media. Phys. Fluids A Fluid Dyn. 1993, 5, 1344–1355. [Google Scholar] [CrossRef] [Green Version]
- Kumagai, A.; Yokoyama, C. Viscosity of Aqueous Solutions of CO2 at High Pressures. Int. J. Thermophys. 1998, 19, 1315–1323. [Google Scholar] [CrossRef]
- Kumagai, A.; Yokoyama, C. Viscosities of Aqueous NaCl Solutions Containing CO2 at High Pressures. J. Chem. Eng. Data 1999, 44, 227–229. [Google Scholar] [CrossRef]
- Bando, S.; Takemura, F.; Nishio, M.; Hihara, E.; Akai, M. Viscosity of Aqueous NaCl Solutions with Dissolved CO2 at (30 to 60) °C and (10 to 20) MPa. J. Chem. Eng. Data 2004, 49, 1328–1332. [Google Scholar] [CrossRef]
- Jha, B.; Cueto-Felgueroso, L.; Juanes, R. Fluid Mixing from Viscous Fingering. Phys. Rev. Lett. 2011, 106, 194502. [Google Scholar] [CrossRef] [PubMed]
- Jha, B.; Cueto-Felgueroso, L.; Juanes, R. Quantifying mixing in viscously unstable porous media flows. Phys. Rev. E 2011, 84, 066312. [Google Scholar] [CrossRef]
- González, D.; Asuaje, M. Simulation of Viscous Fingering Phenomenon Using CFD Tools. In Proceedings of the ASME 2014 International Mechanical Engineering Congress and Exposition, Montreal, QC, Canada, 14–20 November 2014. [Google Scholar]
- Escala, D.M.; De Wit, A.; Carballido-Landeira, J.; Muñuzuri, A.P. Viscous Fingering Induced by a pH-Sensitive Clock Reaction. Langmuir 2019, 35, 4182–4188. [Google Scholar] [CrossRef] [PubMed]
- Nijjer, J.S.; Hewitt, D.R.; Neufeld, J.A. Horizontal miscible displacements through porous media: The interplay between viscous fingering and gravity segregation. J. Fluid Mech. 2022, 935, A14. [Google Scholar] [CrossRef]
- Meulenbroek, B.; Farajzadeh, R.; Bruining, H. The effect of interface movement and viscosity variation on the stability of a diffusive interface between aqueous and gaseous CO2. Phys. Fluids 2013, 25, 074103. [Google Scholar] [CrossRef] [Green Version]
- Daniel, D.; Riaz, A. Effect of viscosity contrast on gravitationally unstable diffusive layers in porous media. Phys. Fluids 2014, 26, 116601. [Google Scholar] [CrossRef]
- Kim, M.C.; Yadav, D. Linear and Nonlinear Analyses of the Onset of Buoyancy-Induced Instability in an Unbounded Porous Medium Saturated by Miscible Fluids. Transp. Porous Media 2014, 104, 407–433. [Google Scholar] [CrossRef]
- Jackson, S.J.; Krevor, S. Small-Scale Capillary Heterogeneity Linked to Rapid Plume Migration During CO2 Storage. Geophys. Res. Lett. 2020, 47, e2020GL088616. [Google Scholar] [CrossRef]
- Morais, S.; Cario, A.; Liu, N.; Bernard, D.; Lecoutre, C.; Garrabos, Y.; Ranchou-Peyruse, A.; Dupraz, S.; Azaroual, M.; Hartman, R.L.; et al. Studying key processes related to CO2 underground storage at the pore scale using high pressure micromodels. React. Chem. Eng. 2020, 5, 1156–1185. [Google Scholar] [CrossRef]
- Singh, M.; Chaudhuri, A.; Stauffer, P.H.; Pawar, R.J. Simulation of Gravitational Instability and Thermo-Solutal Convection During the Dissolution of CO in Deep Storage Reservoirs. Water Resour. Res. 2020, 56, e2019WR026126. [Google Scholar] [CrossRef]
- Chevalier, S.; Faisal, T.F.; Bernabe, Y.; Juanes, R.; Sassi, M. Numerical sensitivity analysis of density driven CO2 convection with respect to different modeling and boundary conditions. Heat Mass Transf. 2015, 51, 941–952. [Google Scholar] [CrossRef]
- Martinez, M.J.; Hesse, M.A. Two-phase convective CO2 dissolution in saline aquifers. Water Resour. Res. 2016, 52, 585–599. [Google Scholar] [CrossRef] [Green Version]
- Emami-Meybodi, H.; Hassanzadeh, H. Two-phase convective mixing under a buoyant plume of CO2 in deep saline aquifers. Adv. Water Resour. 2015, 76, 55–71. [Google Scholar] [CrossRef]
- Elenius, M.T.; Nordbotten, J.M.; Kalisch, H. Effects of a capillary transition zone on the stability of a diffusive boundary layer. IMA J. Appl. Math. 2012, 77, 771–787. [Google Scholar] [CrossRef]
- Emami Meybodi, H.; Hassanzadeh, H. Stability analysis of two-phase buoyancy-driven flow in the presence of a capillary transition zone. Phys. Rev. E 2013, 87, 033009. [Google Scholar] [CrossRef]
- Kim, M.C. The Effect of Boundary Conditions on the Onset of Buoyancy-Driven Convection in a Brine-Saturated Porous Medium. Transp. Porous Media 2015, 107, 469–487. [Google Scholar] [CrossRef]
- Emami-Meybodi, H. Stability analysis of dissolution-driven convection in porous media. Phys. Fluids 2017, 29, 014102. [Google Scholar] [CrossRef]
- Marle, C.M. On macroscopic equations governing multiphase flow with diffusion and chemical reactions in porous media. Int. J. Eng. Sci. 1982, 20, 643–662. [Google Scholar] [CrossRef]
- Chapter 6 Numerical Solution of Two-Phase Flow Problems. In Developments in Petroleum Science; Peaceman, D.W. (Ed.) Elsevier: New York, NY, USA, 1977; Volume 6, pp. 139–168. [Google Scholar]
- Brooks, R.H.; Corey, A.T. Hydraulic Properties of Porous Media and Their Relation to Drainage Design. Trans. ASAE 1964, 7, 26–28. [Google Scholar] [CrossRef]
- Zhang, R.; Yin, X.; Winterfeld, P.H.; Wu, Y.-S. A fully coupled thermal-hydrological-mechanical-chemical model for CO2 geological sequestration. J. Nat. Gas Sci. Eng. 2016, 28, 280–304. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.; Chaudhuri, A.; Chu, S.P.; Stauffer, P.H.; Pawar, R.J. Analysis of evolving capillary transition, gravitational fingering, and dissolution trapping of CO2 in deep saline aquifers during continuous injection of supercritical CO2. Int. J. Greenh. Gas Control 2019, 82, 281–297. [Google Scholar] [CrossRef]
- Juanes, R.; Spiteri, E.J.; Orr Jr, F.M.; Blunt, M.J. Impact of relative permeability hysteresis on geological CO2 storage. Water Resour. Res. 2006, 42, W12418. [Google Scholar] [CrossRef]
- Hidalgo, J.J.; Carrera, J.; Medina, A. Role of salt sources in density-dependent flow. Water Resour. Res. 2009, 45, W05503. [Google Scholar] [CrossRef]
- Riaz, A.; Cinar, Y. Carbon dioxide sequestration in saline formations: Part I—Review of the modeling of solubility trapping. J. Pet. Sci. Eng. 2014, 124, 367–380. [Google Scholar] [CrossRef]
- Slim, A.C.; Ramakrishnan, T.S. Onset and cessation of time-dependent, dissolution-driven convection in porous media. Phys. Fluids 2010, 22, 124103. [Google Scholar] [CrossRef]
- Slim, A.C. Solutal-convection regimes in a two-dimensional porous medium. J. Fluid Mech. 2014, 741, 461–491. [Google Scholar] [CrossRef]
- Zhang, F.; Emami-Meybodi, H. Instability of a Diffusive Boundary Layer beneath a Capillary Transition Zone. Fluids 2018, 3, 85. [Google Scholar] [CrossRef] [Green Version]
- Bachu, S.; Gunter, W.D.; Perkins, E.H. Aquifer disposal of CO2: Hydrodynamic and mineral trapping. Energy Convers. Manag. 1994, 35, 269–279. [Google Scholar] [CrossRef]
- Szulczewski, M.L.; MacMinn, C.W.; Herzog, H.J.; Juanes, R. Lifetime of carbon capture and storage as a climate-change mitigation technology. Proc. Natl. Acad. Sci. USA 2012, 109, 5185–5189. [Google Scholar] [CrossRef] [Green Version]
- Hassanzadeh, H.; Pooladi-Darvish, M.; Keith, D.W. The effect of natural flow of aquifers and associated dispersion on the onset of buoyancy-driven convection in a saturated porous medium. AIChE J. 2009, 55, 475–485. [Google Scholar] [CrossRef]
- Emami-Meybodi, H.; Hassanzadeh, H.; Ennis-King, J. CO2 dissolution in the presence of background flow of deep saline aquifers. Water Resour. Res. 2015, 51, 2595–2615. [Google Scholar] [CrossRef]
- Unwin, H.J.T.; Wells, G.N.; Woods, A.W. CO2 dissolution in a background hydrological flow. J. Fluid Mech. 2016, 789, 768–784. [Google Scholar] [CrossRef] [Green Version]
- Emami-Meybodi, H. Dispersion-driven instability of mixed convective flow in porous media. Phys. Fluids 2017, 29, 094102. [Google Scholar] [CrossRef]
- Nghiem, L.; Yang, C.; Shrivastava, V.; Kohse, B.; Hassam, M.; Card, C. Risk mitigation through the optimization of residual gas and solubility trapping for CO2 storage in saline aquifers. Energy Procedia 2009, 1, 3015–3022. [Google Scholar] [CrossRef] [Green Version]
- Herring, A.L.; Andersson, L.; Wildenschild, D. Enhancing residual trapping of supercritical CO2 via cyclic injections. Geophys. Res. Lett. 2016, 43, 9677–9685. [Google Scholar] [CrossRef] [Green Version]
- Erfani, H.; Babaei, M.; Berg, C.F.; Niasar, V. Scaling CO2 convection in confined aquifers: Effects of dispersion, permeability anisotropy and geochemistry. Adv. Water Resour. 2022, 164, 104191. [Google Scholar] [CrossRef]
- Teng, Y.; Lu, G.; Fan, Y.; Liu, Y.; Jiang, L.; Wang, D.; Song, Y. Experimental Study of Density-driven Convection in Porous Media by Using MRI. Energy Procedia 2017, 105, 4210–4215. [Google Scholar] [CrossRef]
- Teng, Y.; Jiang, L.; Fan, Y.; Liu, Y.; Wang, D.; Abudula, A.; Song, Y. Quantifying the dynamic density driven convection in high permeability packed beds. Magn. Reson. Imaging 2017, 39, 168–174. [Google Scholar] [CrossRef]
- Ching, J.-H.; Chen, P.; Tsai, P.A. Convective mixing in homogeneous porous media flow. Phys. Rev. Fluids 2017, 2, 014102. [Google Scholar] [CrossRef]
- Amarasinghe, W.; Fjelde, I.; Rydland, J.-Å.; Guo, Y. Effects of permeability on CO2 dissolution and convection at reservoir temperature and pressure conditions: A visualization study. Int. J. Greenh. Gas Control 2020, 99, 103082. [Google Scholar] [CrossRef]
- Lengler, U.; De Lucia, M.; Kühn, M. The impact of heterogeneity on the distribution of CO2: Numerical simulation of CO2 storage at Ketzin. Int. J. Greenh. Gas Control 2010, 4, 1016–1025. [Google Scholar] [CrossRef]
- Dykstra, H.; Parsons, R. The Prediction of Oil Recovery by Waterflooding in Secondary Recovery of Oil in the United States., 2nd ed.; American Petroleum Institute: Washington, DC, USA, 1950. [Google Scholar]
- Mirzaei-Paiaman, A.; Asadolahpour, S.R.; Saboorian-Jooybari, H.; Chen, Z.; Ostadhassan, M. A new framework for selection of representative samples for special core analysis. Pet. Res. 2020, 5, 210–226. [Google Scholar] [CrossRef]
- Bestehorn, M.; Firoozabadi, A. Effect of fluctuations on the onset of density-driven convection in porous media. Phys. Fluids 2012, 24, 114102. [Google Scholar] [CrossRef]
- Farajzadeh, R.; Ranganathan, P.; Zitha, P.L.J.; Bruining, J. The effect of heterogeneity on the character of density-driven natural convection of CO2 overlying a brine layer. Adv. Water Resour. 2011, 34, 327–339. [Google Scholar] [CrossRef]
- Chen, C.; Zeng, L.; Shi, L. Continuum-scale convective mixing in geological CO2 sequestration in anisotropic and heterogeneous saline aquifers. Adv. Water Resour. 2013, 53, 175–187. [Google Scholar] [CrossRef]
- Ranganathan, P.; Farajzadeh, R.; Bruining, H.; Zitha, P.L.J. Numerical Simulation of Natural Convection in Heterogeneous Porous media for CO2 Geological Storage. Transp. Porous Media 2012, 95, 25–54. [Google Scholar] [CrossRef] [Green Version]
- Kong, X.-Z.; Saar, M.O. Numerical study of the effects of permeability heterogeneity on density-driven convective mixing during CO2 dissolution storage. Int. J. Greenh. Gas Control 2013, 19, 160–173. [Google Scholar] [CrossRef]
- Green, C.P.; Ennis-King, J. Steady Flux Regime During Convective Mixing in Three-Dimensional Heterogeneous Porous Media. Fluids 2018, 3, 58. [Google Scholar] [CrossRef] [Green Version]
- Elenius, M.T.; Gasda, S.E. Convective mixing in formations with horizontal barriers. Adv. Water Resour. 2013, 62, 499–510. [Google Scholar] [CrossRef]
- Hong, J.S.; Kim, M.C. Effect of anisotropy of porous media on the onset of buoyancy-driven convection. Transp. Porous Media 2008, 72, 241–253. [Google Scholar] [CrossRef]
- Rapaka, S.; Pawar, R.J.; Stauffer, P.H.; Zhang, D.; Chen, S. Onset of convection over a transient base-state in anisotropic and layered porous media. J. Fluid Mech. 2009, 641, 227–244. [Google Scholar] [CrossRef] [Green Version]
- Myint, P.C.; Bestehorn, M.; Firoozabadi, A. Effect of permeability anisotropy on buoyancy-driven flow for CO2 sequestration in saline aquifers. Water Resour. Res. 2012, 48, W09539. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Chen, S.; Zhang, D. Convective stability analysis of the long-term storage of carbon dioxide in deep saline aquifers. Adv. Water Resour. 2006, 29, 397–407. [Google Scholar] [CrossRef]
- Soltanian, M.R.; Amooie, M.A.; Gershenzon, N.; Dai, Z.; Ritzi, R.; Xiong, F.; Cole, D.; Moortgat, J. Dissolution Trapping of Carbon Dioxide in Heterogeneous Aquifers. Environ. Sci. Technol. 2017, 51, 7732–7741. [Google Scholar] [CrossRef]
- Abbaszadeh, M.; Shariatipour, S.M. Investigating the Impact of Reservoir Properties and Injection Parameters on Carbon Dioxide Dissolution in Saline Aquifers. Fluids 2018, 3, 76. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Cai, W.; Tang, X.; Chen, Y.; Li, B.; Chen, C.-Y. The impact of heterogeneous anisotropy of porous media on density-driven convection. Int. J. Numer. Methods Heat Fluid Flow 2020, 30, 956–976. [Google Scholar] [CrossRef]
- Sun, Y.; Payton, R.L.; Hier-Majumder, S.; Kingdon, A. Geological Carbon Sequestration by Reactive Infiltration Instability. Front. Earth Sci. 2020, 8, 533588. [Google Scholar] [CrossRef]
- Gasow, S.; Lin, Z.; Zhang, H.C.; Kuznetsov, A.V.; Avila, M.; Jin, Y. Effects of pore scale on the macroscopic properties of natural convection in porous media. J. Fluid Mech. 2020, 891, A25. [Google Scholar] [CrossRef]
- Gasow, S.; Kuznetsov, A.V.; Avila, M.; Jin, Y. A macroscopic two-length-scale model for natural convection in porous media driven by a species-concentration gradient. J. Fluid Mech. 2021, 926, A8. [Google Scholar] [CrossRef]
- Aggelopoulos, C.A.; Tsakiroglou, C.D. The effect of micro-heterogeneity and capillary number on capillary pressure and relative permeability curves of soils. Geoderma 2008, 148, 25–34. [Google Scholar] [CrossRef]
- Ozgur, E.; Gumrah, F. Analytical and Numerical Modeling of CO2 Sequestration in Deep Saline Aquifers. Energy Sources Part A: Recovery Util. Environ. Eff. 2010, 32, 674–687. [Google Scholar] [CrossRef]
- Ozgur, E.; Gumrah, F. Diffusive and Convective Mechanisms during CO2 Sequestration in Aquifers. Energy Sources Part A: Recovery Util. Environ. Eff. 2009, 31, 698–709. [Google Scholar] [CrossRef]
- Beni, A.N.; Kuehn, M.; Meyer, R.; Clauser, C. Numerical Modeling of a Potential Geological CO2 Sequestration Site at Minden (Germany). Environ. Model. Assess. 2012, 17, 337–351. [Google Scholar] [CrossRef]
- Islam, A.; Sun, A.Y.; Yang, C. Reactive Transport Modeling of the Enhancement of Density-Driven CO2 Convective Mixing in Carbonate Aquifers and its Potential Implication on Geological Carbon Sequestration. Sci. Rep. 2016, 6, 24768. [Google Scholar] [CrossRef] [Green Version]
- Jensen, J.L.; Lake, L.W. The Influence of Sample Size and Permeability Distribution on Heterogeneity Measures. Spe Reserv. Eng. 1988, 3, 629–637. [Google Scholar] [CrossRef]
- Zhang, K.; Moridis, G.; Pruess, K. TOUGH+CO2: A multiphase fluid-flow simulator for CO2 geologic sequestration in saline aquifers. Comput. Geosci. 2011, 37, 714–723. [Google Scholar] [CrossRef]
- Peysson, Y. Permeability alteration induced by drying of brines in porous media. Eur. Phys. J. Appl. Phys. 2012, 60, 24206. [Google Scholar] [CrossRef]
- Saaltink, M.W.; Vilarrasa, V.; De Gaspari, F.; Silva, O.; Carrera, J.; Roetting, T.S. A method for incorporating equilibrium chemical reactions into multiphase flow models for CO2 storage. Adv. Water Resour. 2013, 62, 431–441. [Google Scholar] [CrossRef]
- Sung, R.-T.; Li, M.-H.; Dong, J.-J.; Lin, A.T.-S.; Hsu, S.-K.; Wang, C.-Y.; Yang, C.-N. Numerical assessment of CO2 geological sequestration in sloping and layered heterogeneous formations: A case study from Taiwan. Int. J. Greenh. Gas Control 2014, 20, 168–179. [Google Scholar] [CrossRef]
- Zhang, R.; Winterfeld, P.H.; Yin, X.; Wu, Y.-S.; Xiong, Y. Coupled geomechanical and reactive geochemical model for fluid, heat flow and convective mixing: Application for CO2 geological sequestration into saline aquifer with heterogeneity. Int. J. Glob. Warm. 2017, 13, 197–236. [Google Scholar] [CrossRef]
- Sainz-Garcia, A.; Abarca, E.; Nardi, A.; Grandia, F.; Oelkers, E.H. Convective mixing fingers and chemistry interaction in carbon storage. Int. J. Greenh. Gas Control 2017, 58, 52–61. [Google Scholar] [CrossRef] [Green Version]
- Brouzet, C.; Meheust, Y.; Meunier, P. CO2 convective dissolution in a three-dimensional granular porous medium: An experimental study. Phys. Rev. Fluids 2022, 7, 033802. [Google Scholar] [CrossRef]
- Tilton, N. Onset of transient natural convection in porousmedia due to porosity perturbations. J. Fluid Mech. 2018, 838, 129–147. [Google Scholar] [CrossRef]
- Han, W.S.; Lee, S.-Y.; Lu, C.; McPherson, B.J. Effects of permeability on CO2trapping mechanisms and buoyancy-driven CO2migration in saline formations. Water Resour. Res. 2010, 46, W07510. [Google Scholar] [CrossRef]
- Iding, M.; Ringrose, P. Evaluating the impact of fractures on the performance of the In Salah CO2 storage site. Int. J. Greenh. Gas Control 2010, 4, 242–248. [Google Scholar] [CrossRef]
- Nguyen, M.; Onishi, T.; Carey, J.W.; Will, B.; Zaluski, W.; Bowen, D.; DeVault, B.; Duguid, A.; Spangler, L.; Stauffer, P.H. Risk Assessment of Carbon Sequestration into A Naturally Fractured Reservoir at Kevin Dome; Los Alamos National Laboratory: Los Alamos, NM, USA, 2017; No. LA-UR-17-31501. [Google Scholar]
- Kim, K.-Y.; Oh, J.; Han, W.S.; Park, K.G.; Shinn, Y.J.; Park, E. Two-phase flow visualization under reservoir conditions for highly heterogeneous conglomerate rock: A core-scale study for geologic carbon storage. Sci. Rep. 2018, 8, 4869. [Google Scholar] [CrossRef] [Green Version]
- Middleton, R.; Viswanathan, H.; Currier, R.; Gupta, R. CO2 as a fracturing fluid: Potential for commercial-scale shale gas production and CO2 sequestration. Energy Procedia 2014, 63, 7780–7784. [Google Scholar] [CrossRef] [Green Version]
- Yao, L.; Zhai, M.; Wang, S. A Numerical Investigation on the Hydraulic Fracturing Efficiency in Radial Well. Geotech. Geol. Eng. 2019, 37, 4503–4513. [Google Scholar] [CrossRef]
- Cherubini, C. A Modeling Approach for the Study of Contamination in a Fractured Aquifer. Geotech. Geol. Eng. 2008, 26, 519–533. [Google Scholar] [CrossRef]
- Renu, V.; Suresh Kumar, G. Numerical Modeling and Spatial Moment Analysis of Solute Mobility and Spreading in a Coupled Fracture-Skin-Matrix System. Geotech. Geol. Eng. 2012, 30, 1289–1302. [Google Scholar] [CrossRef]
- Sekhar, M.; Kumar, G.S. Modelling Transport of Linearly Sorbing Solutes in a Single Fracture: Asymptotic Behavior of Solute Velocity and Dispersivity. Geotech. Geol. Eng. 2006, 24, 183–201. [Google Scholar] [CrossRef]
- Mortezaei, K.; Vahedifard, F. Numerical Simulation of Induced Seismicity in Carbon Capture and Storage Projects. Geotech. Geol. Eng. 2015, 33, 411–424. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, S.; Jiang, W.; Wang, Z.; Li, J.; Bian, Y. A mechanism of fluid exchange associated to CO2 leakage along activated fault during geologic storage. Energy 2018, 165, 1178–1190. [Google Scholar] [CrossRef]
- Tang, D.H.; Frind, E.O.; Sudicky, E.A. Contaminant transport in fractured porous media: Analytical solution for a single fracture. Water Resour. Res. 1981, 17, 555–564. [Google Scholar] [CrossRef]
- Graf, T.; Therrien, R. Variable-density groundwater flow and solute transport in porous media containing nonuniform discrete fractures. Adv. Water Resour. 2005, 28, 1351–1367. [Google Scholar] [CrossRef]
- Iding, M.; Blunt, M.J. Enhanced solubility trapping of CO2 in fractured reservoirs. Energy Procedia 2011, 4, 4961–4968. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Kim, K.Y.; Han, W.S.; Oh, J.; Park, E. Density-Driven Convection in a Fractured Porous Media: Implications for Geological CO2 Storage. Water Resour. Res. 2019, 55, 5852–5870. [Google Scholar] [CrossRef]
- Shikaze, S.G.; Sudicky, E.A.; Schwartz, F.W. Density-dependent solute transport in discretely-fractured geologic media: Is prediction possible? J. Contam. Hydrol. 1998, 34, 273–291. [Google Scholar] [CrossRef]
- Graf, T.; Therrien, R. Variable-density groundwater flow and solute transport in irregular 2D fracture networks. Adv. Water Resour. 2007, 30, 455–468. [Google Scholar] [CrossRef]
- Vujević, K.; Graf, T.; Simmons, C.T.; Werner, A.D. Impact of fracture network geometry on free convective flow patterns. Adv. Water Resour. 2014, 71, 65–80. [Google Scholar] [CrossRef]
- Shafabakhsh, P.; Fahs, M.; Ataie-Ashtiani, B.; Simmons, C.T. Unstable Density-Driven Flow in Fractured Porous Media: The Fractured Elder Problem. Fluids 2019, 4, 168. [Google Scholar] [CrossRef] [Green Version]
- Vujević, K.; Graf, T. Combined inter- and intra-fracture free convection in fracture networks embedded in a low-permeability matrix. Adv. Water Resour. 2015, 84, 52–63. [Google Scholar] [CrossRef]
- Rutqvist, J. The Geomechanics of CO2 Storage in Deep Sedimentary Formations. Geotech. Geol. Eng. 2012, 30, 525–551. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Kordi, M.; Hovorka, S.D.; Meckel, T.A.; Christopher, C.A. Reservoir characterization and complications for trapping mechanisms at Cranfield CO2 injection site. Int. J. Greenh. Gas Control 2013, 18, 361–374. [Google Scholar] [CrossRef]
- Cavanagh, A.; Nazarian, B. A new and extended Sleipner Benchmark model for CO2 storage simulations in the Utsira Formation. Energy Procedia 2014, 63, 2831–2835. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Nakanishi, Y.; Hyodo, A.; Suekane, T. Three-dimensional Finger Structure of Natural Convection in Homogeneous and Heterogeneous Porous Medium. Energy Procedia 2017, 114, 5048–5057. [Google Scholar] [CrossRef]
- Farajzadeh, R.; Zinati, F.F.; Zitha, P.; Bruining, J. Density driven natural convection in dual layered and anisotropic porous media with application for CO2 injection projects. In Proceedings of the 11th European Conference on the Mathematics of Oil Recovery (ECMOR X1), Bergen, Norway, 8–11 September 2008; pp. 8–11. [Google Scholar]
- Taheri, A.; Wessel-Berg, D.; Torsaeter, O.; Soroush, M. The Effects of Anisotropy and Heterogeneity on CO2 Dissolution in Deep Saline Aquifers. In Proceedings of the Carbon Management Technology Conference, Orlando, FL, USA, 7–9 February 2012; p. CMTC–151345-MS. [Google Scholar]
- Taheri, A.; Torsæter, O.; Lindeberg, E.; Hadia, N.; Wessel-Berg, D. Effect of Convective Mixing Process on Storage of CO2; in Saline Aquifers with Layered Permeability. Adv. Chem. Res. 2021, 3, 12. [Google Scholar] [CrossRef]
- Agartan, E.; Trevisan, L.; Cihan, A.; Birkholzer, J.; Zhou, Q.; Illangasekare, T.H. Experimental study on effects of geologic heterogeneity in enhancing dissolution trapping of supercritical CO2. Water Resour. Res. 2015, 51, 1635–1648. [Google Scholar] [CrossRef]
- Wang, S.; Cheng, Z.; Jiang, L.; Song, Y.; Liu, Y. Quantitative study of density-driven convection mass transfer in porous media by MRI. J. Hydrol. 2021, 594, 125941. [Google Scholar] [CrossRef]
- Tsai, P.A.; Riesing, K.; Stone, H.A. Density-driven convection enhanced by an inclined boundary: Implications for geological CO2 storage. Phys. Rev. E 2013, 87, 011003. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Cheng, Z.; Zhang, Y.; Jiang, L.; Liu, Y.; Song, Y. Unstable Density-Driven Convection of CO2 in Homogeneous and Heterogeneous Porous Media with Implications for Deep Saline Aquifers. Water Resour. Res. 2021, 57, e2020WR028132. [Google Scholar] [CrossRef]
- Lv, P.; Liu, Y.; Chen, J.; Jiang, L.; Wu, B.; Liu, S.; Song, Y. Pore-scale investigation of effects of heterogeneity on CO2 geological storage using stratified sand packs. Greenh. Gases Sci. Technol. 2017, 7, 972–987. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, D. Pore-scale simulation of density-driven convection in fractured porous media during geological CO2 sequestration. Water Resour. Res. 2010, 46, W11527. [Google Scholar] [CrossRef]
- White, S.P.; Allis, R.G.; Moore, J.; Chidsey, T.; Morgan, C.; Gwynn, W.; Adams, M. Simulation of reactive transport of injected CO2 on the Colorado Plateau, Utah, USA. Chem. Geol. 2005, 217, 387–405. [Google Scholar] [CrossRef]
- Wheatley, D.; Hollingworth, S.; Steele, P.; Chan, M. Sedimentology, diagenesis, and reservoir characterization of the Permian White Rim Sandstone, southern Utah: Implications for carbon capture and sequestration potential. AAPG Bull. 2020, 104, 1357–1373. [Google Scholar] [CrossRef]
- Hintze, L.F. Geologic history of Utah. In Brigham Young University Geology Studies/Special Publication; Brigham Young University: Provo, UT, USA, 1988; Volume 7, 202p. [Google Scholar]
- Chen, J.; Jia, S.; Zhang, X. Back Analysis and Application of Confined Aquifer Hydrogeological Parameters Based on Pumping Test. Geotech. Geol. Eng. 2017, 35, 2851–2861. [Google Scholar] [CrossRef]
- Akinnikawe, O.; Ehlig-Economides, C.A. Geologic model and fluid flow simulation of Woodbine aquifer CO2 sequestration. Int. J. Greenh. Gas Control 2016, 49, 1–13. [Google Scholar] [CrossRef]
- Alexander, D.; Boodlal, D. Evaluating the effects of CO2 Injection in Faulted Saline Aquifers. Energy Procedia 2014, 63, 3012–3021. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, B.-Z.; Nghiem, L.; Shen, C.-H.; Lin, Z.-S. Effects of complex sandstone-shale sequences of a storage formation on the risk of CO2 leakage: Case study from Taiwan. Int. J. Greenh. Gas Control 2013, 17, 376–387. [Google Scholar] [CrossRef]
- Ghanbari, S.; Al-Zaabi, Y.; Pickup, G.E.; Mackay, E.; Gozalpour, F.; Todd, A.C. Simulation of CO2 storage in saline aquifers. Chem. Eng. Res. Des. 2006, 84, 764–775. [Google Scholar] [CrossRef] [Green Version]
- Vilarrasa, V.; Carrera, J.; Bolster, D.; Dentz, M. Semianalytical Solution for CO2 Plume Shape and Pressure Evolution During CO2 Injection in Deep Saline Formations. Transp. Porous Media 2013, 97, 43–65. [Google Scholar] [CrossRef] [Green Version]
- MacMinn, C.W.; Juanes, R. Buoyant currents arrested by convective dissolution. Geophys. Res. Lett. 2013, 40, 2017–2022. [Google Scholar] [CrossRef]
- Gelhar, L.W.; Welty, C.; Rehfeldt, K.R. A critical review of data on field-scale dispersion in aquifers. Water Resour. Res. 1992, 28, 1955–1974. [Google Scholar] [CrossRef]
- Nikolaevskii, V.N. Convective diffusion in porous media. J. Appl. Math. Mech. 1959, 23, 1492–1503. [Google Scholar] [CrossRef]
- Bear, J. On the tensor form of dispersion in porous media. J. Geophys. Res. 1961, 66, 1185–1197. [Google Scholar] [CrossRef]
- Scheidegger, A.E. General theory of dispersion in porous media. J. Geophys. Res. 1961, 66, 3273–3278. [Google Scholar] [CrossRef]
- Aggelopoulos, C.A.; Tsakiroglou, C.D. Effects of micro-heterogeneity and hydrodynamic dispersion on the dissolution rate of carbon dioxide in water-saturated porous media. Int. J. Greenh. Gas Control 2012, 10, 341–350. [Google Scholar] [CrossRef]
- Bear, J.; Bachmat, Y. Introduction to Modeling of Transport Phenomena in Porous Media; Springer Science & Business Media: Berlin, Germany, 2012; Volume 4. [Google Scholar]
- Hidalgo, J.J.; Carrera, J. Effect of dispersion on the onset of convection during CO2 sequestration. J. Fluid Mech. 2009, 640, 441–452. [Google Scholar] [CrossRef] [Green Version]
- Ghesmat, K.; Hassanzadeh, H.; Abedi, J. The effect of anisotropic dispersion on the convective mixing in long-term CO2 storage in saline aquifers. AIChE J. 2011, 57, 561–570. [Google Scholar] [CrossRef]
- Xie, Y.; Simmons, C.T.; Werner, A.D. Speed of free convective fingering in porous media. Water Resour. Res. 2011, 47, W11501. [Google Scholar] [CrossRef]
- Dhar, J.; Meunier, P.; Nadal, F.; Meheust, Y. Convective dissolution of carbon dioxide in two- and three-dimensional porous media: The impact of hydrodynamic dispersion. Phys. Fluids 2022, 34, 064114. [Google Scholar] [CrossRef]
- Menand, T.; Woods, A.W. Dispersion, scale, and time dependence of mixing zones under gravitationally stable and unstable displacements in porous media. Water Resour. Res. 2005, 41, W05014. [Google Scholar] [CrossRef]
- Liang, Y.; Wen, B.; Hesse, M.A.; DiCarlo, D. Effect of Dispersion on Solutal Convection in Porous Media. Geophys. Res. Lett. 2018, 45, 9690–9698. [Google Scholar] [CrossRef]
- Wang, L.; Nakanishi, Y.; Hyodo, A.; Suekane, T. Three-dimensional structure of natural convection in a porous medium: Effect of dispersion on finger structure. Int. J. Greenh. Gas Control 2016, 53, 274–283. [Google Scholar] [CrossRef]
- Wang, L.; Cai, S.; Suekane, T. Gravitational Fingering Due to Density Increase by Mixing at a Vertical Displacing Front in Porous Media. Energy Fuels 2018, 32, 658–669. [Google Scholar] [CrossRef]
- Nakanishi, Y.; Hyodo, A.; Wang, L.; Suekane, T. Experimental study of 3D Rayleigh-Taylor convection between miscible fluids in a porous medium. Adv. Water Resour. 2016, 97, 224–232. [Google Scholar] [CrossRef]
- Bharath, K.S.; Sahu, C.K.; Flynn, M.R. Isolated buoyant convection in a two-layered porous medium with an inclined permeability jump. J. Fluid Mech. 2020, 902, A22. [Google Scholar] [CrossRef]
- Tóth, J. A theoretical analysis of groundwater flow in small drainage basins. J. Geophys. Res. 1963, 68, 4795–4812. [Google Scholar] [CrossRef]
- Jiang, X.-W.; Wan, L.; Cardenas, M.B.; Ge, S.; Wang, X.-S. Simultaneous rejuvenation and aging of groundwater in basins due to depth-decaying hydraulic conductivity and porosity. Geophys. Res. Lett. 2010, 37, L05403. [Google Scholar] [CrossRef] [Green Version]
- Cserepes, L.; Lenkey, L. Forms of hydrothermal and hydraulic flow in a homogeneous unconfined aquifer. Geophys. J. Int. 2004, 158, 785–797. [Google Scholar] [CrossRef] [Green Version]
- Fang, Y.; Zheng, T.; Wang, H.; Zheng, X.; Walther, M. Influence of Dynamically Stable-Unstable Flow on Seawater Intrusion and Submarine Groundwater Discharge Over Tidal and Seasonal Cycles. J. Geophys. Res. Ocean. 2022, 127, e2021JC018209. [Google Scholar] [CrossRef]
- Zhang, X.; Jiao, J.J.; Li, H.; Luo, X.; Kuang, X. Effects of Downward Intrusion of Saline Water on Nested Groundwater Flow Systems. Water Resour. Res. 2020, 56, e2020WR028377. [Google Scholar] [CrossRef]
- Michel-Meyer, I.; Shavit, U.; Tsinober, A.; Rosenzweig, R. The Role of Water Flow and Dispersive Fluxes in the Dissolution of CO2 in Deep Saline Aquifers. Water Resour. Res. 2020, 56, e2020WR028184. [Google Scholar] [CrossRef]
- Tsinober, A.; Rosenzweig, R.; Class, H.; Helmig, R.; Shavit, U. The Role of Mixed Convection and Hydrodynamic Dispersion During CO2 Dissolution in Saline Aquifers: A Numerical Study. Water Resour. Res. 2022, 58, e2021WR030494. [Google Scholar] [CrossRef]
- van Eldik, R.; Palmer, D.A. Effects of pressure on the kinetics of the dehydration of carbonic acid and the hydrolysis of CO2 in aqueous solution. J. Solut. Chem. 1982, 11, 339–346. [Google Scholar] [CrossRef]
- Nighswander, J.A.; Kalogerakis, N.; Mehrotra, A.K. Solubilities of carbon dioxide in water and 1 wt. % sodium chloride solution at pressures up to 10 MPa and temperatures from 80 to 200 °C. J. Chem. Eng. Data 1989, 34, 355–360. [Google Scholar] [CrossRef]
- Ennis-King, J.; Paterson, L. Engineering Aspects of Geological Sequestration of Carbon Dioxide. In Proceedings of the SPE Asia Pacific Oil and Gas Conference and Exhibition, Melbourne, VIC, Australia, 8–10 October 2002; p. SPE–77809-MS. [Google Scholar]
- Diamond, L.W.; Akinfiev, N.N. Solubility of CO2 in water from −1.5 to 100 °C and from 0.1 to 100 MPa: Evaluation of literature data and thermodynamic modelling. Fluid Phase Equilibria 2003, 208, 265–290. [Google Scholar] [CrossRef]
- Pruess, K.; Xu, T.; Apps, J.; Garcia, J. Numerical modeling of aquifer disposal of CO2. SPE J. 2003, 8, 49–60. [Google Scholar] [CrossRef]
- Benson, S.M.; Cole, D.R. CO2 Sequestration in Deep Sedimentary Formations. Elements 2008, 4, 325–331. [Google Scholar] [CrossRef]
- Hassanzadeh, H.; Pooladi-Darvish, M.; Keith, D.W. Accelerating CO2 Dissolution in Saline Aquifers for Geological Storage—Mechanistic and Sensitivity Studies. Energy Fuels 2009, 23, 3328–3336. [Google Scholar] [CrossRef]
- Yan, W.; Huang, S.; Stenby, E.H. Measurement and modeling of CO2 solubility in NaCl brine and CO2–saturated NaCl brine density. Int. J. Greenh. Gas Control 2011, 5, 1460–1477. [Google Scholar] [CrossRef]
- Ji, Y.; Ji, X.; Lu, X.; Tu, Y. Modeling mass transfer of CO2 in brine at high pressures by chemical potential gradient. Sci. China Chem. 2013, 56, 821–830. [Google Scholar] [CrossRef] [Green Version]
- Mao, S.; Zhang, D.; Li, Y.; Liu, N. An improved model for calculating CO2 solubility in aqueous NaCl solutions and the application to CO2–H2O–NaCl fluid inclusions. Chem. Geol. 2013, 347, 43–58. [Google Scholar] [CrossRef]
- Loodts, V.; Rongy, L.; De Wit, A. Impact of pressure, salt concentration, and temperature on the convective dissolution of carbon dioxide in aqueous solutions. Chaos Interdiscip. J. Nonlinear Sci. 2014, 24, 043120. [Google Scholar] [CrossRef] [Green Version]
- Mojtaba, S.; Behzad, R.; Rasoul, N.M.; Mohammad, R. Experimental study of density-driven convection effects on CO2 dissolution rate in formation water for geological storage. J. Nat. Gas Sci. Eng. 2014, 21, 600–607. [Google Scholar] [CrossRef]
- Nomeli, M.A.; Tilton, N.; Riaz, A. A new model for the density of saturated solutions of CO2–H2O–NaCl in saline aquifers. Int. J. Greenh. Gas Control 2014, 31, 192–204. [Google Scholar] [CrossRef]
- Zhang, D.; Song, J. Mechanisms for Geological Carbon Sequestration. Procedia IUTAM 2014, 10, 319–327. [Google Scholar] [CrossRef] [Green Version]
- Seyyedi, M.; Rostami, B.; Pasdar, M.; Pazhoohan, J. Experimental and numerical study of the effects of formation brine salinity and reservoir temperature on convection mechanism during CO2 storage in saline aquifers. J. Nat. Gas Sci. Eng. 2016, 36, 950–962. [Google Scholar] [CrossRef]
- Ajayi, T.; Gomes, J.S.; Bera, A. A review of CO2 storage in geological formations emphasizing modeling, monitoring and capacity estimation approaches. Pet. Sci. 2019, 16, 1028–1063. [Google Scholar] [CrossRef] [Green Version]
- Ajayi, T.; Awolayo, A.; Gomes, J.S.; Parra, H.; Hu, J. Large scale modeling and assessment of the feasibility of CO2 storage onshore Abu Dhabi. Energy 2019, 185, 653–670. [Google Scholar] [CrossRef]
- Kumar, R.; Campbell, S.; Sonnenthal, E.; Cunningham, J. Effect of brine salinity on the geological sequestration of CO2 in a deep saline carbonate formation. Greenh. Gases Sci. Technol. 2020, 10, 296–312. [Google Scholar] [CrossRef]
- Nazari Moghaddam, R.; Rostami, B.; Pourafshary, P. Scaling Analysis of the Convective Mixing in Porous Media for Geological Storage of CO2: An Experimental Approach. Chem. Eng. Commun. 2015, 202, 815–822. [Google Scholar] [CrossRef]
- Teng, Y.; Wang, P.; Jiang, L.; Liu, Y.; Wei, Y. New Spectrophotometric Method for Quantitative Characterization of Density-Driven Convective Instability. Polymers 2021, 13, 611. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wang, Y.; Lu, G.; Yang, J.; Song, Y. Experimental Study on the Density-Driven Convective Mixing of CO2 and Brine at Reservoir Temperature and Pressure Conditions. Energy Fuels 2022, 36, 10261–10268. [Google Scholar] [CrossRef]
- Szulczewski, M.L.; MacMinn, C.W.; Juanes, R. Theoretical analysis of how pressure buildup and CO2 migration can both constrain storage capacity in deep saline aquifers. Int. J. Greenh. Gas Control 2014, 23, 113–118. [Google Scholar] [CrossRef]
- Abbaszadeh, M.; Shariatipour, S.M. Enhancing CO2 solubility in the aquifer with the use of a downhole cooler tools. Int. J. Greenh. Gas Control 2020, 97, 103039. [Google Scholar] [CrossRef]
- Wan, Y.; Du, S.; Lin, G.; Zhang, F.; Xu, T. Dissolution sequestration mechanism of CO2 at the Shiqianfeng saline aquifer in the Ordos Basin, northwestern China. Arab. J. Geosci. 2017, 10, 71. [Google Scholar] [CrossRef]
- Rosenbauer, R.J.; Koksalan, T.; Palandri, J.L. Experimental investigation of CO2–brine–rock interactions at elevated temperature and pressure: Implications for CO2 sequestration in deep-saline aquifers. Fuel Process. Technol. 2005, 86, 1581–1597. [Google Scholar] [CrossRef]
- Jafari Raad, S.M.; Azin, R.; Osfouri, S. Measurement of CO2 diffusivity in synthetic and saline aquifer solutions at reservoir conditions: The role of ion interactions. Heat Mass Transf. 2015, 51, 1587–1595. [Google Scholar] [CrossRef]
- Messabeb, H.; Contamine, F.; Cézac, P.; Serin, J.P.; Pouget, C.; Gaucher, E.C. Experimental Measurement of CO2 Solubility in Aqueous CaCl2 Solution at Temperature from 323.15 to 423.15 K and Pressure up to 20 MPa Using the Conductometric Titration. J. Chem. Eng. Data 2017, 62, 4228–4234. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, F.; Xu, J.; Qi, Y. Experimental Investigation and Numerical Simulation of CO2–Brine–Rock Interactions during CO2 Sequestration in a Deep Saline Aquifer. Sustainability 2019, 11, 317. [Google Scholar] [CrossRef] [Green Version]
- Mahmoodpour, S.; Rostami, B.; Soltanian, M.R.; Amooie, M.A. Convective Dissolution of Carbon Dioxide in Deep Saline Aquifers: Insights from Engineering a High-Pressure Porous Visual Cell. Phys. Rev. Appl. 2019, 12, 034016. [Google Scholar] [CrossRef] [Green Version]
- Edem, D.E.; Abba, M.K.; Nourian, A.; Babaie, M.; Naeem, Z. Experimental Study on the Interplay between Different Brine Types/Concentrations and CO2 Injectivity for Effective CO2 Storage in Deep Saline Aquifers. Sustainability 2022, 14, 986. [Google Scholar] [CrossRef]
- Koornneef, J.; Ramírez, A.; Turkenburg, W.; Faaij, A. The environmental impact and risk assessment of CO2 capture, transport and storage-an evaluation of the knowledge base using the DPSIR framework. Energy Procedia 2011, 4, 2293–2300. [Google Scholar] [CrossRef] [Green Version]
- Koornneef, J.; Ramírez, A.; Turkenburg, W.; Faaij, A. The environmental impact and risk assessment of CO2 capture, transport and storage—An evaluation of the knowledge base. Prog. Energy Combust. Sci. 2012, 38, 62–86. [Google Scholar] [CrossRef]
- Cichosz, M.; Kiełkowska, U.; Skowron, K.; Kiedzik, Ł.; Łazarski, S.; Szkudlarek, M.; Kowalska, B.; Żurawski, D. Changes in Synthetic Soda Ash Production and Its Consequences for the Environment. Materials 2022, 15, 4828. [Google Scholar] [CrossRef]
- Basava-Reddi, L.; Wildgust, N.; Ryan, D. Effects of Impurities on Geological Storage of Carbon Dioxide. In Proceedings of the 1st EAGE Sustainable Earth Sciences (SES) Conference and Exhibition, 8–11 November 2011; European Association of Geoscientists & Engineers: Bunnik, The Netherlands, 2011; p. cp–268-00052. [Google Scholar]
- Talman, S. Subsurface geochemical fate and effects of impurities contained in a CO2 stream injected into a deep saline aquifer: What is known. Int. J. Greenh. Gas Control 2015, 40, 267–291. [Google Scholar] [CrossRef]
- Hajiw, M.; Corvisier, J.; El Ahmar, E.; Coquelet, C. Impact of impurities on CO2 storage in saline aquifers: Modelling of gases solubility in water. Int. J. Greenh. Gas Control 2018, 68, 247–255. [Google Scholar] [CrossRef]
- Yu, Y.; Li, Y.; Cheng, F.; Yang, G.; Ma, X.; Cao, W. Effects of impurities H2S and N2 on CO2 migration and dissolution in sedimentary geothermal reservoirs. J. Hydrol. 2021, 603, 126959. [Google Scholar] [CrossRef]
- Ji, X.; Zhu, C. Predicting Possible Effects of H2S Impurity on CO2 Transportation and Geological Storage. Environ. Sci. Technol. 2013, 47, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Jiang, X. A numerical study of the impurity effects of nitrogen and sulfur dioxide on the solubility trapping of carbon dioxide geological storage. Appl. Energy 2014, 128, 60–74. [Google Scholar] [CrossRef]
- Li, D.; Jiang, X. An investigation of chromatographic partitioning of CO2 and multiple impurities in geological CO2 sequestration. Energy Procedia 2015, 75, 2240–2245. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Jiang, X.; Meng, Q.; Xie, Q. Numerical analyses of the effects of nitrogen on the dissolution trapping mechanism of carbon dioxide geological storage. Comput. Fluids 2015, 114, 1–11. [Google Scholar] [CrossRef]
- Kim, M.C.; Song, K.H. Effect of impurities on the onset and growth of gravitational instabilities in a geological CO2 storage process: Linear and nonlinear analyses. Chem. Eng. Sci. 2017, 174, 426–444. [Google Scholar] [CrossRef]
- Li, D.; Jiang, X. Numerical investigation of convective mixing in impure CO2 geological storage into deep saline aquifers. Int. J. Greenh. Gas Control 2020, 96, 103015. [Google Scholar] [CrossRef]
- Ennis-King, J.; Paterson, L. Role of Convective Mixing in the Long-Term Storage of Carbon Dioxide in Deep Saline Formations. SPE J. 2005, 10, 349–356. [Google Scholar] [CrossRef]
- Kather, A.; Kownatzki, S. Assessment of the different parameters affecting the CO2 purity from coal fired oxyfuel process. Int. J. Greenh. Gas Control 2011, 5, S204–S209. [Google Scholar] [CrossRef]
- Jafari Raad, S.M.; Hassanzadeh, H. Onset of dissolution-driven instabilities in fluids with nonmonotonic density profile. Phys. Rev. E 2015, 92, 053023. [Google Scholar] [CrossRef] [PubMed]
- Jafari Raad, S.M.; Emami-Meybodi, H.; Hassanzadeh, H. On the choice of analogue fluids in CO2 convective dissolution experiments. Water Resour. Res. 2016, 52, 4458–4468. [Google Scholar] [CrossRef]
- Jafari Raad, S.M.; Hassanzadeh, H.; Ennis-King, J. On the Dynamics of Two-Component Convective Dissolution in Porous Media. Water Resour. Res. 2019, 55, 4030–4042. [Google Scholar] [CrossRef]
- Omrani, S.; Mahmoodpour, S.; Rostami, B.; Salehi Sedeh, M.; Sass, I. Diffusion coefficients of CO2–SO2–water and CO2–N2–water systems and their impact on the CO2 sequestration process: Molecular dynamics and dissolution process simulations. Greenh. Gases Sci. Technol. 2021, 11, 764–779. [Google Scholar] [CrossRef]
- Mahmoodpour, S.; Rostami, B.; Emami-Meybodi, H. Onset of convection controlled by N2 impurity during CO2 storage in saline aquifers. Int. J. Greenh. Gas Control 2018, 79, 234–247. [Google Scholar] [CrossRef]
- Mahmoodpour, S.; Amooie, M.A.; Rostami, B.; Bahrami, F. Effect of gas impurity on the convective dissolution of CO2 in porous media. Energy 2020, 199, 117397. [Google Scholar] [CrossRef]
- Raad, S.M.J.; Hassanzadeh, H. Prospect for storage of impure carbon dioxide streams in deep saline aquifers-A convective dissolution perspective. Int. J. Greenh. Gas Control 2017, 63, 350–355. [Google Scholar] [CrossRef]
- Raad, S.M.J.; Hassanzadeh, H. Does impure CO2 impede or accelerate the onset of convective mixing in geological storage? Int. J. Greenh. Gas Control 2016, 54, 250–257. [Google Scholar] [CrossRef]
- Jafari Raad, S.M.; Hassanzadeh, H. Comments on the paper “effect of impurities on the onset and the growth of gravitational instabilities in a geological CO2 storage process: Linear and nonlinear analyses” M.C. Kim, K.H. Song (2017). Chem. Eng. Sci. 2018, 192, 613–618. [Google Scholar] [CrossRef]
- Kim, M.C.; Song, K.H. Responses to the comment on the paper “Effect of impurities on the onset and the growth of gravitational instabilities in a geological CO2 storage process: Linear and nonlinear analyses” by M.C. Kim and K.H. Song. Chem. Eng. Sci. 2019, 193, 184–187. [Google Scholar] [CrossRef]
- Wei, N.; Li, X.; Wang, Y.; Wang, Y.; Kong, W. Numerical study on the field-scale aquifer storage of CO2 containing N2. Energy Procedia 2013, 37, 3952–3959. [Google Scholar] [CrossRef] [Green Version]
- Bachu, S.; Bennion, D.B. Chromatographic partitioning of impurities contained in a CO2 stream injected into a deep saline aquifer: Part 1. Effects of gas composition and in situ conditions. Int. J. Greenh. Gas Control 2009, 3, 458–467. [Google Scholar] [CrossRef]
- Bachu, S.; Pooladi-Darvish, M.; Hong, H. Chromatographic partitioning of impurities (H2S) contained in a CO2 stream injected into a deep saline aquifer: Part 2. Effects of flow conditions. Int. J. Greenh. Gas Control 2009, 3, 468–473. [Google Scholar] [CrossRef]
- Wei, N.; Li, X.C. Numerical studies on the aquifer storage of CO2 containing N2. Energy Procedia 2011, 4, 4314–4322. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; He, Y.; Zhang, H.; Xu, W.; Jiang, X. A numerical study of the impurity effects on CO2 geological storage in layered formation. Appl. Energy 2017, 199, 107–120. [Google Scholar] [CrossRef]
- Pooladi-Darvish, M.; Hong, H.; Stocker, R.K.; Bennion, B.; Theys, S.; Bachu, S. Chromatographic Partitioning of H2S and CO2 in Acid Gas Disposal. J. Can. Pet. Technol. 2009, 48, 52–57. [Google Scholar] [CrossRef]
- Li, D.; Jiang, X. Numerical investigation of the partitioning phenomenon of carbon dioxide and multiple impurities in deep saline aquifers. Appl. Energy 2017, 185, 1411–1423. [Google Scholar] [CrossRef] [Green Version]
- Bacon, D.H.; Ramanathan, R.; Schaef, H.T.; McGrail, B.P. Simulating geologic co-sequestration of carbon dioxide and hydrogen sulfide in a basalt formation. Int. J. Greenh. Gas Control 2014, 21, 165–176. [Google Scholar] [CrossRef]
- Choi, B.Y.; Shin, Y.-J.; Park, Y.-C.; Park, J. Preliminary results of numerical simulation in a small-scale CO2 injection pilot site: 2. Effect of SO2 impurity on CO2 storage. J. Geol. Soc. Korea 2015, 51, 497. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Chen, S.; Li, D.; Jiang, X. Density-Driven Convection for CO2 Solubility Trapping in Saline Aquifers: Modeling and Influencing Factors. Geotechnics 2023, 3, 70-103. https://doi.org/10.3390/geotechnics3010006
Chen Y, Chen S, Li D, Jiang X. Density-Driven Convection for CO2 Solubility Trapping in Saline Aquifers: Modeling and Influencing Factors. Geotechnics. 2023; 3(1):70-103. https://doi.org/10.3390/geotechnics3010006
Chicago/Turabian StyleChen, Yizhen, Suihong Chen, Didi Li, and Xi Jiang. 2023. "Density-Driven Convection for CO2 Solubility Trapping in Saline Aquifers: Modeling and Influencing Factors" Geotechnics 3, no. 1: 70-103. https://doi.org/10.3390/geotechnics3010006