Metal–Support Interaction of Carbon–Based Electrocatalysts for Oxygen Evolution Reaction
Abstract
:1. Introduction
2. Fundamentals of the Oxygen Evolution Reaction

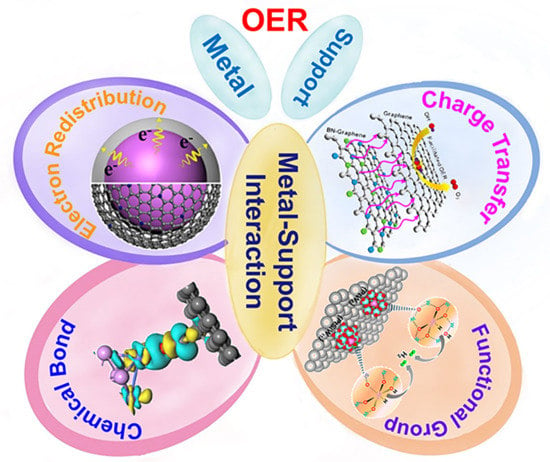
3. Metal–Support Interaction Effect
3.1. Stabilization Effect

3.2. Electronic Structure Regulation
3.2.1. Metal–Carbon Electron Transfer
3.2.2. Redistribution of Electron Density
3.3. Geometric Structure Modulation
3.3.1. Coordination by Functional Groups
3.3.2. Chemical Covalent Bonding
4. Modulation Strategies of Metal–Support Interaction
4.1. Metal Properties
4.1.1. Particle Size
4.1.2. Metal Morphology
4.2. Carbon Support
4.2.1. Heteroatom Doping
4.2.2. Defect Engineering
4.3. Coordination Effect
4.3.1. Coordination Atoms

4.3.2. Coordination Number
5. Conclusions and Perspectives
- (1)
- Improving intrinsic catalytic activity and atom utilization efficiency. Decreasing metal size from nanoparticles to single atoms can expose more active sites and improve atom utilization efficiency. Changing the coordination atoms, coordination number, and local chemical environment can regulate the intrinsic activity of active centers.
- (2)
- Controllable adjustment of MSI to change OER mechanisms. The covalent state of the M-O bond can be altered by MSI via charge transfer and electron redistribution, shifting the OER mechanism from AEM to LOM. Difficulties in controllable modulation of MSI through a simple process hampers the application of MSI in reaction mechanism optimization.
- (3)
- Enhancing the stability of electrocatalysts during the OER process. Catalytic activity and stability are mutually restricted for most reported catalysts. Carbon–supported catalysts can improve the dispersion of metal nanoparticles and modulate MSI strength of the electron coupling between carbon supports and metals for enhanced stability.
- (4)
- Developing advanced characterization techniques. Limited characterization techniques including in situ/operando experimental studies and theoretical calculations inhibit the accurate analysis of MSI across the interfaces of catalysts. The development of advanced detection methods and simulation techniques to realize in situ analysis in catalytic processes is conducive to understanding the mechanism and effects of MSI.

Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ren, C.; Lu, S.; Wu, Y.; Ouyang, Y.; Zhang, Y.; Li, Q.; Ling, C.; Wang, J. A universal descriptor for complicated interfacial effects on electrochemical reduction reactions. J. Am. Chem. Soc. 2022, 144, 12874–12883. [Google Scholar] [CrossRef]
- Gao, Y.; Xue, Y.; Qi, L.; Xing, C.; Zheng, X.; He, F.; Li, Y. Rhodium nanocrystals on porous graphdiyne for electrocatalytic hydrogen evolution from saline water. Nat. Commun. 2022, 13, 5227. [Google Scholar] [CrossRef]
- Luo, W.; Wang, Y.; Luo, L.; Gong, S.; Wei, M.; Li, Y.; Gan, X.; Zhao, Y.; Zhu, Z.; Li, Z. Single-atom and bimetallic nanoalloy supported on nanotubes as a bifunctional electrocatalyst for ultrahigh-current-density overall water splitting. ACS Catal. 2022, 12, 1167–1179. [Google Scholar] [CrossRef]
- Li, J.-C.; Meng, Y.; Zhang, L.; Li, G.; Shi, Z.; Hou, P.-X.; Liu, C.; Cheng, H.-M.; Shao, M. Dual-phasic carbon with Co single atoms and nanoparticles as a bifunctional oxygen electrocatalyst for rechargeable Zn-air batteries. Adv. Funct. Mater. 2021, 31, 2103360. [Google Scholar] [CrossRef]
- Yuan, S.; Peng, J.; Cai, B.; Huang, Z.; Garcia-Esparza, A.T.; Sokaras, D.; Zhang, Y.; Giordano, L.; Akkiraju, K.; Zhu, Y.G.; et al. Tunable metal hydroxide–organic frameworks for catalysing oxygen evolution. Nat. Mater. 2022, 21, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Suo, H.; Zheng, X.; Zhang, T.; Lei, Y.; Wang, Y.-X.; Lai, W.-H.; Wang, G. Lightest metal leads to big change: Lithium-mediated metal oxides for oxygen evolution reaction. Adv. Energy Mater. 2022, 12, 2201934. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, C.; Zhao, S.-N.; Wu, Y.; Liu, C.; Huang, J.; Xue, L.; Sun, J.; Zhang, W.; Wang, X.; et al. A dual-site doping strategy for developing efficient perovskite oxide electrocatalysts towards oxygen evolution reaction. Nano Energy 2022, 99, 107344. [Google Scholar] [CrossRef]
- Zhang, Y.-C.; Han, C.; Gao, J.; Pan, L.; Wu, J.; Zhu, X.-D.; Zou, J.-J. NiCo-based electrocatalysts for the alkaline oxygen evolution reaction: A review. ACS Catal. 2021, 11, 12485–12509. [Google Scholar] [CrossRef]
- Xu, X.; Pan, Y.; Zhong, Y.; Shi, C.; Guan, D.; Ge, L.; Hu, Z.; Chin, Y.-Y.; Lin, H.-J.; Chen, C.-T.; et al. New undisputed evidence and strategy for enhanced lattice-oxygen participation of perovskite electrocatalyst through cation deficiency manipulation. Adv. Sci. 2022, 9, 2200530. [Google Scholar] [CrossRef]
- Lu, M.; Zhang, X.; Yang, F.; Wang, L.; Wang, Y. Surface/interface modulation in oxygen evolution reaction. Prog. Chem. 2022, 34, 547–556. [Google Scholar]
- Song, L.; Zhang, X.; Zhu, S.; Xu, Y.; Wang, Y. Hydrogen spillover effect enhanced by carbon quantum dots activated MoS2. Carbon 2022, 199, 63–69. [Google Scholar] [CrossRef]
- Zhang, X.; Song, L.; Tong, L.; Zeng, M.; Wang, Y. Surface bonding of CoP to biomass derived carbon microtube: Site-specific growth and high-efficiency catalysis. Chem. Eng. J. 2022, 440, 135884. [Google Scholar] [CrossRef]
- Song, L.; Zhang, X.; Zhu, S.; Xu, Y.; Wang, Y. Transition metal (Fe, Ni, and Zn) doping-induced modulation of geometric and electronic structures to optimize the potential-determining step of MnCo2O4 for oxygen evolution reaction. Sci. China Mater. 2022, 65, 2871–2878. [Google Scholar] [CrossRef]
- Ding, J.; Song, L.; Li, X.; Chen, L.; Li, X.; Sun, J.; Zhang, X.; Wang, Y.; Tian, X. Interfacial engineering of the platinum/molybdenum disulfide/graphitic carbon nitride composite for enhanced photocatalytic hydrogen production. ACS Appl. Energy Mater. 2022, 5, 8800–8811. [Google Scholar] [CrossRef]
- Li, L.; Song, L.; Zhang, X.; Zhu, S.; Wang, Y. Effect of substitutional and interstitial boron-doped NiCo2S4 on the electronic structure and surface adsorption: High rate and long-term stability. ACS Appl. Energy Mater. 2022, 5, 2505–2513. [Google Scholar] [CrossRef]
- Song, L.; Zhang, X.; Du, X.; Zhu, S.; Xu, Y.; Wang, Y. Rapid surface reconstruction strategy for oxygen evolution reaction: Chem-grafted MXene quantum dots on Ni-Co layered double hydroxide. Phys. Chem. Chem. Phys. 2022, 24, 24902–24909. [Google Scholar] [CrossRef]
- Zhang, K.; Zou, R. Advanced transition metal-based OER electrocatalysts: Current status, opportunities, and challenges. Small 2021, 17, 2100129. [Google Scholar] [CrossRef]
- Li, P.; Wan, X.; Su, J.; Liu, W.; Guo, Y.; Yin, H.; Wang, D. A single-phase FeCoNiMnMo high-entropy alloy oxygen evolution anode working in alkaline solution for over 1000 h. ACS Catal. 2022, 12, 11667–11674. [Google Scholar] [CrossRef]
- Wang, C.; Zhai, P.; Xia, M.; Wu, Y.; Zhang, B.; Li, Z.; Ran, L.; Gao, J.; Zhang, X.; Fan, Z.; et al. Engineering lattice oxygen activation of iridium clusters stabilized on amorphous bimetal borides array for oxygen evolution reaction. Angew. Chem. Int. Ed. 2021, 133, 27332–27340. [Google Scholar] [CrossRef]
- Peng, M.; Huang, J.; Zhu, Y.; Zhou, H.; Hu, Z.; Liao, Y.-K.; Lai, Y.-H.; Chen, C.-T.; Chu, Y.-H.; Zhang, K.H.L.; et al. Structural anisotropy determining the oxygen evolution mechanism of strongly correlated perovskite nickelate electrocatalyst. ACS Sustain. Chem. Eng. 2021, 9, 4262–4270. [Google Scholar] [CrossRef]
- Liao, X.; Lu, R.; Xia, L.; Liu, Q.; Wang, H.; Zhao, K.; Wang, Z.; Zhao, Y. Density functional theory for electrocatalysis. Energy Environ. Mater. 2022, 5, 157–185. [Google Scholar] [CrossRef]
- Li, C.; Liu, F.; Shi, Y.; Zheng, Y.; Fang, B.; Lin, J.; Ni, J.; Wang, X.; Lin, B.; Jiang, L. Inducing the metal-support interaction and enhancing the ammonia synthesis activity of ceria-supported ruthenium catalyst via N2H4 reduction. ACS Sustain. Chem. Eng. 2021, 9, 4885–4893. [Google Scholar] [CrossRef]
- Qiao, Z.; Wang, C.; Zeng, Y.; Spendelow, J.S.; Wu, G. Advanced nanocarbons for enhanced performance and durability of platinum catalysts in proton exchange membrane fuel cells. Small 2021, 17, 2006805. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Xie, Y.; Gao, X.; Li, L.; Lin, Z. Simultaneous optimization of CoIr alloy nanoparticles and 2D graphitic-N doped carbon support in CoIr@CN by Ir doping for enhanced oxygen and hydrogen evolution reactions. J. Mater. Chem. A 2022, 10, 15543–15553. [Google Scholar] [CrossRef]
- Wang, R.; Liu, B.; You, S.; Li, Y.; Zhang, Y.; Wang, D.; Tang, B.; Sun, Y.; Zou, J. Three-dimensional Ni3Se4 flowers integrated with ultrathin carbon layer with strong electronic interactions for boosting oxygen reduction/evolution reactions. Chem. Eng. J. 2022, 430, 132720. [Google Scholar] [CrossRef]
- Jayabal, S.; Saranya, G.; Geng, D.; Lin, L.-Y.; Meng, X. Insight into the correlation of Pt–support interactions with electrocatalytic activity and durability in fuel cells. J. Mater. Chem. A 2020, 8, 9420–9446. [Google Scholar] [CrossRef]
- Liang, Z.; Kong, N.; Yang, C.; Zhang, W.; Zheng, H.; Lin, H.; Cao, R. Highly curved nanostructure-coated Co, N-doped carbon materials for oxygen electrocatalysis. Angew. Chem. Int. Ed. 2021, 60, 12759–12764. [Google Scholar] [CrossRef]
- Huang, M.; Liu, S.; Gong, S.; Xu, P.; Yang, K.; Chen, S.; Wang, C.; Chen, Q. Silver nanoparticles encapsulated in an N-doped porous carbon matrix as high-active catalysts toward oxygen reduction reaction via electron transfer to outer graphene shells. ACS Sustain. Chem. Eng. 2019, 7, 16511–16519. [Google Scholar] [CrossRef]
- Rong, C.; Shen, X.; Wang, Y.; Thomsen, L.; Zhao, T.; Li, Y.; Lu, X.; Amal, R.; Zhao, C. Electronic structure engineering of single-atom Ru sites via Co-N4 sites for bifunctional pH-universal water splitting. Adv. Mater. 2022, 34, 2110103. [Google Scholar] [CrossRef]
- Chen, D.; Zhu, J.; Mu, X.; Cheng, R.; Li, W.; Liu, S.; Pu, Z.; Lin, C.; Mu, S. Nitrogen-doped carbon coupled FeNi3 intermetallic compound as advanced bifunctional electrocatalyst for OER, ORR and Zn-air batteries. Appl. Catal. B Environ. 2020, 268, 118729. [Google Scholar] [CrossRef]
- Riyajuddin, S.; Pahuja, M.; Sachdeva, P.K.; Azmi, K.; Kumar, S.; Afshan, M.; Ali, F.; Sultana, J.; Maruyama, T.; Bera, C.; et al. Super-hydrophilic leaflike Sn4P3 on the porous seamless graphene-carbon nanotube heterostructure as an efficient electrocatalyst for solar-driven overall water splitting. ACS Nano 2022, 16, 4861–4875. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Z.; Wang, K.; Dai, Q.; Lei, C.; Yang, B.; Zhang, Q.; Lei, L.; Leunge, M.K.H.; Hou, Y. Tuning d-band center of tungsten carbide via Mo doping for efficient hydrogen evolution and Zn–H2O cell over a wide pH range. Nano Energy 2020, 74, 104850. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, Z.; Lei, Z.; Yu, L.; Wu, W.; Wang, Z.; Cheng, N. Electronic modulation optimizes OH* intermediate adsorption on Co-Nx-C sites via coupling CoNi alloy in hollow carbon nanopolyhedron toward efficient reversible oxygen electrocatalysis. Appl. Catal. B Environ. 2022, 304, 121006. [Google Scholar] [CrossRef]
- Fu, J.; Lym, J.; Zheng, W.; Alexopoulos, K.; Mironenko, A.V.; Li, N.; Boscoboinik, J.A.; Su, D.; Weber, R.T.; Vlachos, D.G. C–O bond activation using ultralow loading of noble metal catalysts on moderately reducible oxides. Nat. Catal. 2020, 3, 446–453. [Google Scholar] [CrossRef]
- Gang, C.; Chen, J.; Chen, Q.; Chen, Y. Heterostructure of ultrafine FeOOH nanodots supported on CoAl-layered double hydroxide nanosheets as highly efficient electrocatalyst for water oxidation. J. Colloid Interf. Sci. 2021, 600, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Yang, B.; Li, A.; Liao, C.; Chen, G.; Liu, M.; Liu, X.; Ma, R.; Zhang, N. Tuning interfacial active sites over porous Mo2N-supported cobalt sulfides for efficient hydrogen evolution reactions in acid and alkaline electrolytes. ACS Appl. Mater. Interfaces 2021, 13, 41573–41583. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Tian, M.; Wang, H.; Wei, C.; Sun, Z.; Rummeli, M.H.; Strasser, P.; Sun, J.; Yang, R. Mildly oxidized MXene (Ti3C2, Nb2C, and V2C) electrocatalyst via a generic strategy enables longevous Li-O2 Battery under a high rate. ACS Nano 2021, 15, 19640–19650. [Google Scholar] [CrossRef]
- Suryawanshi, M.P.; Ghorpade, U.V.; Shin, S.W.; Suryawanshi, U.P.; Jo, E.; Kim, J.H. Hierarchically coupled Ni: FeOOH nanosheets on 3D N-doped graphite foam as self-supported electrocatalysts for efficient and durable water oxidation. ACS Catal. 2019, 9, 5025–5034. [Google Scholar] [CrossRef]
- Liang, D.; Lian, C.; Xu, Q.; Liu, M.; Liu, H.; Jiang, H.; Li, C. Interfacial charge polarization in Co2P2O7@N, P co-doped carbon nanocages as Mott-Schottky electrocatalysts for accelerating oxygen evolution reaction. Appl. Catal. B Environ. 2020, 268, 118417. [Google Scholar] [CrossRef]
- Pan, Y.; Ma, X.; Wang, M.; Yang, X.; Liu, S.; Chen, H.-C.; Zhuang, Z.; Zhang, Y.; Cheong, W.-C.; Zhang, C.; et al. Construction of N, P co-doped carbon frames anchored with Fe single atoms and Fe2P nanoparticles as a robust coupling catalyst for electrocatalytic oxygen reduction. Adv. Mater. 2022, 34, 2203621. [Google Scholar] [CrossRef]
- Zhang, K.; Min, X.; Zhang, T.; Si, M.; Jiang, J.; Chai, L.; Shi, Y. Biodeposited nano-CdS drives the in situ growth of highly dispersed sulfide nanoparticles during pyrolysis for enhanced oxygen evolution reaction. ACS Appl. Mater. Interfaces 2020, 12, 54553–54562. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Morales, I.; Reyes-Carmona, A.; Dupont, M.; Cavaliere, S.; Rodlert, M.; Mornaghini, F.; Larsen, M.J.; Odgaard, M.; Zajac, J.; Jones, D.J.; et al. Correlation between the surface characteristics of carbon supports and their electrochemical stability and performance in fuel cell cathode. Carbon Energy 2021, 3, 654–665. [Google Scholar] [CrossRef]
- Su, J.; Yuan, S.; Cheng, Y.-X.; Yang, Z.-M.; Zuo, J.-L. Coordination-bond-directed synthesis of hydrogen-bonded organic frameworks from metal–organic frameworks as templates. Chem. Sci. 2021, 12, 14254–14259. [Google Scholar] [CrossRef] [PubMed]
- 4Jiang, B.; Fan, X.; Dang, Q.; Liao, F.; Li, Y.; Lin, H.; Kang, Z.; Shao, M. Functionalization of metal oxides with thiocyanate groups: A general strategy for boosting oxygen evolution reaction in neutral media. Nano Energy 2020, 76, 105079. [Google Scholar]
- Zhang, Y.; Yang, J.; Yu, Z.; Hou, Y.; Jiang, R.; Huang, J.; Yang, F.; Yao, S.; Gao, L.; Tang, W. Modulating carbon-supported transition metal oxide by electron-giving and electron-absorbing functional groups towards efficient overall water splitting. Chem. Eng. J. 2021, 416, 129124. [Google Scholar] [CrossRef]
- Andaveh, R.; Darband, G.B.; Maleki, M.; Rouhaghdam, A.S. Superaerophobic/superhydrophilic surfaces as advanced electrocatalysts for the hydrogen evolution reaction: A comprehensive review. J. Mater. Chem. A 2022, 10, 5147–5173. [Google Scholar] [CrossRef]
- Yin, H.; Xia, H.; Zhao, S.; Li, K.; Zhang, J.; Mu, S. Atomic level dispersed metal-nitrogen-carbon catalyst toward oxygen reduction reaction: Synthesis strategies and chemical environmental regulation. Energy Environ. Mater. 2021, 4, 5–18. [Google Scholar] [CrossRef]
- Yin, P.; Luo, X.; Ma, Y.; Chu, S.-Q.; Chen, S.; Zheng, X.; Lu, J.; Wu, X.-J.; Liang, H.-W. Sulfur stabilizing metal nanoclusters on carbon at high temperatures. Nat. Commun. 2021, 12, 3135. [Google Scholar] [CrossRef]
- Zhong, X.; Huang, K.; Zhang, Y.; Wang, Y.; Feng, S. Constructed interfacial oxygen-bridge chemical bonding in core-shell transition metal phosphides/carbon hybrid boosting oxygen evolution reaction. ChemSusChem 2021, 14, 2188–2197. [Google Scholar] [CrossRef]
- Wang, J.; Zeng, H.C. Hybrid OER electrocatalyst combining mesoporous hollow spheres of N, P-doped carbon with ultrafine Co2NiOx. ACS Appl. Mater. Interfaces 2020, 12, 50324–50332. [Google Scholar] [CrossRef]
- Wang, X.; Raghupathy, R.K.M.; Querebillo, C.J.; Liao, Z.; Li, D.; Lin, K.; Hantusch, M.; Sofer, Z.; Li, B.; Zschech, E.; et al. Interfacial covalent bonds regulated electron-deficient 2D black phosphorus for electrocatalytic oxygen reactions. Adv. Mater. 2021, 33, 2008752. [Google Scholar] [CrossRef]
- Wang, D.; Xin, Y.; Li, X.; Ning, H.; Wang, Y.; Yao, D.; Zheng, Y.; Meng, Z.; Yang, Z.; Pan, Y.; et al. Transforming metal-organic frameworks into porous liquids via a covalent linkage strategy for CO2 capture. ACS Appl. Mater. Interfaces 2021, 13, 2600–2609. [Google Scholar] [CrossRef] [PubMed]
- Akyüz, D.; Şenocak, A.; Köksoy, B.; Ömeroğlu, İ.; Durmuş, M.; Demirbas, E. Coumarin bearing asymmetrical zinc (II) phthalocyanine functionalized SWCNT hybrid nanomaterial. J. Electroanal. Chem. 2021, 897, 115552. [Google Scholar] [CrossRef]
- He, Y.; Shi, Q.; Shan, W.; Li, X.; Kropf, A.J.; Wegener, E.C.; Wright, J.; Karakalos, S.; Su, D.; Cullen, D.A.; et al. Dynamically unveiling metal-nitrogen coordination during thermal activation to design high-efficient atomically dispersed CoN4 active sites. Angew. Chem. Int. Ed. 2021, 60, 9516–9526. [Google Scholar] [CrossRef]
- Lin, G.; Ju, Q.; Jin, Y.; Qi, X.; Liu, W.; Huang, F.; Wang, J. Suppressing dissolution of Pt-based electrocatalysts through the electronic metal-support interaction. Adv. Energy Mater. 2021, 11, 2101050. [Google Scholar] [CrossRef]
- TriKhoa, N.; WookKim, S.; Yoo, D.-H.; Kim, E.J.; HongHahn, S. Size-dependent work function and catalytic performance of gold nanoparticles decorated graphene oxide sheets. Appl. Catal. A Gen. 2014, 469, 159–164. [Google Scholar]
- Wu, M.; Zhang, G.; Wang, W.; Yang, H.; Rawach, D.; Chen, M.; Sun, S. Electronic metal-support interaction modulation of single-atom electrocatalysts for rechargeable zinc-air batteirs. Small Methods 2022, 6, 2100947. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, L.; Cao, Z.; Kozlov, S.M.; García de Arquer, F.P.; Dinh, C.T.; Li, J.; Wang, Z.; Zheng, X.; Zhang, L.; et al. High-valence metals improve oxygen evolution reaction performance by modulating 3d metal oxidation cycle energetics. Nat. Catal. 2020, 3, 985–992. [Google Scholar] [CrossRef]
- Zhao, F.; Wen, B.; Niu, W.; Chen, Z.; Yan, C.; Selloni, A.; Tully, C.G.; Yang, X.; Koel, B.E. Increasing iridium oxide activity for the oxygen evolution reaction with hafnium modification. J. Am. Chem. Soc. 2021, 143, 15616–15623. [Google Scholar] [CrossRef]
- Yang, C.-L.; Wang, L.-N.; Yin, P.; Liu, J.; Chen, M.-X.; Yan, Q.-Q.; Wang, Z.-S.; Xu, S.-L.; Chu, S.-Q.; Cui, C.; et al. Sulfur-anchoring synthesis of platinum intermetallic nanoparticle catalysts for fuel cells. Science 2021, 374, 459–464. [Google Scholar] [CrossRef]
- Li, W.; Zhang, H.; Zhang, K.; Hu, W.; Cheng, Z.; Chen, H.; Feng, X.; Peng, T.; Kou, Z. Monodispersed ruthenium nanoparticles interfacially bonded with defective nitrogen-and-phosphorus-doped carbon nanosheets enable pH-universal hydrogen evolution reaction. Appl. Catal. B Environ. 2022, 306, 121095. [Google Scholar] [CrossRef]
- Sandbeck, D.J.S.; Secher, N.M.; Speck, F.D.; Sørensen, J.E.; Kibsgaard, J.; Chorkendorff, I.; Cherevko, S. The particle size effect on platinum dissolution: Considerations for accelerated stability testing of fuel cell catalysts. ACS Catal. 2020, 10, 6281–6290. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, X.; Tao, B.; Wang, X.; Deng, Y.; Gu, X.; Li, L.; Xiao, W.; Li, N.; Luo, H. N-doped hollow porous carbon spheres@Co Cu Fe alloy nanospheres as novel non-precious metal electrocatalysts for HER and OER. Int. J. Hydrogen Energy 2022, 47, 5947–5960. [Google Scholar]
- Lai, W.-H.; Zhang, L.-F.; Hua, W.-B.; Indris, S.; Yan, Z.-C.; Hu, Z.; Zhang, B.; Liu, Y.; Wang, L.; Liu, M.; et al. General p-electron-assisted strategy for Ir, Pt, Ru, Pd, Fe, Ni single atom electrocatalysts with bifunctional active sites for highly efficient water splitting. Angew. Chem. Int. Ed. 2019, 58, 11868–11873. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Xue, D.; Zhang, J.-N. Optimizing atomically dispersed metal electrocatalysts for hydrogen evolution: Chemical coordination effect and electronic metal support interaction. Chem. Asian J. 2022, 17, e202200319. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Liu, Z.; Li, M.; Tian, J.; Feng, L. Surface structure regulation and evaluation of FeNi-based nanoparticles for oxygen evolution reaction. Appl. Catal. B Environ. 2021, 297, 120462. [Google Scholar] [CrossRef]
- Wei, S.; Cui, X.; Xu, Y.; Shang, B.; Zhang, Q.; Gu, L.; Fan, X.; Zheng, L.; Hou, C.; Huang, H.; et al. Iridium-triggered phase transition of MoS2 nanosheets boosts overall water splitting in alkaline media. ACS Energy Lett. 2019, 4, 368–374. [Google Scholar] [CrossRef]
- Babar, P.; Patil, K.; Karade, V.; Gour, K.; Lokhande, A.; Pawar, S.; Kim, J.H. In situ fabrication of nickel-iron oxalate catalysts for electrochemical water oxidation at high current densities. ACS Appl. Mater. Interfaces 2021, 13, 52620–52628. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, Z.; Xue, Y.; He, F.; Li, Y. Acidic water oxidation on quantum dots of IrOx/graphdiyne. Adv. Energy Mater. 2021, 11, 2101138. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, Q.; Liu, H.; Liang, J.; Peng, W.; Li, Y.; Zhang, F.; Fan, X. Preparation of hollow cobalt-iron phosphides nanospheres by controllable atom migration for enhanced water oxidation and splitting. Small 2021, 17, 2007858. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Zhang, C.; Liu, Z.; Yuan, S.; Yang, G.; Li, N. Ultra-small NiFe-layered double hydroxide nanoparticles confined in ordered mesoporous carbon as efficient electrocatalyst for oxygen evolution reaction. Appl. Surf. Sci. 2021, 565, 150533. [Google Scholar] [CrossRef]
- Wei, G.; Shen, Y.; Zhao, X.; Wang, Y.; Zhang, W.; An, C. Hexagonal phase Ni3Fe nanosheets toward high-performance water splitting by a room-temperature methane plasma method. Adv. Funct. Mater. 2022, 32, 2109709. [Google Scholar] [CrossRef]
- Yang, H.; Dai, G.; Chen, Z.; Wu, J.; Huang, H.; Liu, Y.; Shao, M.; Kang, Z. Pseudo-periodically coupling Ni-O lattice with Ce-O lattice in ultrathin heteronanowire arrays for efficient water oxidation. Small 2021, 17, 2101727. [Google Scholar] [CrossRef]
- Weng, B.; Grice, C.R.; Meng, W.; Guan, L.; Xu, F.; Yu, Y.; Wang, C.; Zhao, D.; Yan, Y. Metal-organic framework-derived CoWP@C composite nanowire electrocatalyst for efficient water splitting. ACS Energy Lett. 2018, 3, 1434–1442. [Google Scholar] [CrossRef]
- Li, B.; Xing, R.; Mohite, S.V.; Latthe, S.S.; Fujishima, A.; Liu, S.; Zhou, Y. CoS2 nanodots anchored into heteroatom-doped carbon layer via a biomimetic strategy: Boosting the oxygen evolution and supercapacitor performance. J. Power Sources 2019, 436, 226862. [Google Scholar] [CrossRef]
- Zhang, M.; Xuan, X.; Wang, W.; Ma, C.; Lin, Z. Anode photovoltage compensation-enabled synergistic CO2 photoelectrocatalytic reduction on a flower-like graphene-decorated Cu foam cathode. Adv. Funct. Mater. 2020, 30, 2005983. [Google Scholar] [CrossRef]
- Cao, D.; Xu, H.; Cheng, D. Branch-leaf-shaped CuNi@NiFeCu nanodendrites as highly efficient electrocatalysts for overall water splitting. Appl. Catal. B Environ. 2021, 298, 120600. [Google Scholar] [CrossRef]
- Yang, B.; Huang, Z.; Wu, H.; Hu, H.; Lin, H.; Nie, M.; Li, Q. Sea urchin-like CoSe2 nanoparticles modified graphene oxide as an efficient and stable hydrogen evolution catalyst. J. Electroanal. Chem. 2022, 907, 116037. [Google Scholar] [CrossRef]
- Li, Z.; Lin, X.; Xi, W.; Shen, M.; Gao, B.; Chen, Y.; Zheng, Y.; Lin, B. Bamboo-like N, S-doped carbon nanotubes with encapsulated Co nanoparticles as high-performance electrocatalyst for liquid and flexible all-solid-state rechargeable Zn-air batteries. Appl. Surf. Sci. 2022, 593, 153446. [Google Scholar] [CrossRef]
- Yoon, K.R.; Hwang, C.-K.; Kim, S.-H.; Jung, J.-W.; Chae, J.E.; Kim, J.; Lee, K.A.; Lim, A.; Cho, S.-H.; Singh, J.P.; et al. Hierarchically assembled cobalt oxynitride nanorods and N-doped carbon nanofibers for efficient bifunctional oxygen electrocatalysis with exceptional regenerative efficiency. ACS Nano 2021, 15, 11218–11230. [Google Scholar] [CrossRef]
- Xue, W.; Zhou, Q.; Cui, X.; Jia, S.; Zhang, J.; Lin, Z. Metal–organic frameworks-derived heteroatom-doped carbon electrocatalysts for oxygen reduction reaction. Nano Energy 2021, 86, 106073. [Google Scholar] [CrossRef]
- Zhong, X.; Tang, J.; Wang, J.; Shao, M.; Chai, J.; Wang, S.; Yang, M.; Yang, Y.; Wang, N.; Wang, S.; et al. 3D heterostructured pure and N-Doped Ni3S2/VS2 nanosheets for high efficient overall water splitting. Electrochim. Acta 2018, 269, 55–61. [Google Scholar] [CrossRef]
- Jiang, E.; Li, J.; Li, X.; Ali, A.; Wang, G.; Ma, S.; Shen, P.K.; Zhu, J. MoP-Mo2C quantum dot heterostructures uniformly hosted on a heteroatom-doped 3D porous carbon sheet network as an efficient bifunctional electrocatalyst for overall water splitting. Chem. Eng. J. 2022, 431, 133719. [Google Scholar] [CrossRef]
- Kumar, S.S.; Ramakrishna, S.U.B.; Devi, B.R.; Himabindu, V. Phosphorus-doped graphene supported palladium (Pd/PG) electrocatalyst for the hydrogen evolution reaction in PEM water electrolysis. Int. J. Green Energy 2018, 15, 558–567. [Google Scholar] [CrossRef]
- Joshi, P.; Yadav, R.; Hara, M.; Inoue, T.; Motoyama, Y.; Yoshimura, M. Contribution of B, N-co-doped reduced graphene oxide as a catalyst support to the activity of iridium oxide for oxygen evolution reaction. J. Mater. Chem. A 2021, 9, 9066–9080. [Google Scholar] [CrossRef]
- Ma, X.; Chang, C.; Zhang, Y.; Niu, P.; Liu, X.; Wang, S.; Li, L. Synthesis of Co-based prussian blue analogues/dual-doped hollow carbon microsphere hybrids as high-performance bifunctional electrocatalysts for oxygen evolution and overall water splitting. ACS Sustain. Chem. Eng. 2020, 8, 8318–8326. [Google Scholar] [CrossRef]
- Yu, J.; Li, J.; Xu, C.-Y.; Li, Q.; Liu, Q.; Liu, J.; Chen, R.; Zhu, J.; Wang, J. Modulating the d-band centers by coordination environment regulation of single-atom Ni on porous carbon fibers for overall water splitting. Nano Energy 2022, 98, 107266. [Google Scholar] [CrossRef]
- Guo, F.; Liu, Z.; Zhang, Y.; Xiao, J.; Zeng, X.; Zhang, C.; Dong, P.; Liu, T.; Zhang, Y.; Li, M. Tiny Ni nanoparticles embedded in boron- and nitrogen-co doped porous carbon nanowires for high-efficiency water splitting. ACS Appl. Mater. Interfaces 2022, 14, 24447–24461. [Google Scholar] [CrossRef]
- Tian, J.; Wang, Z.; Wang, Y.; Yuan, D.; Tian, F.; Zhang, L. Nitrogen-doped binary spinel CuCo2O4/C nanocomposite: An efficient electrocatalyst for oxygen evolution reaction. ChemNanoMat 2020, 6, 1652–1657. [Google Scholar] [CrossRef]
- Ren, J.-T.; Ying, Y.-D.; Liu, Y.-P.; Li, W.; Yuan, Z.-Y. Charge redistribution caused by sulfur doping of bimetal FeCo phosphides supported on heteroatoms-doped graphene for Zn-air batteries with stable cycling. J. Energy Chem. 2022, 71, 619–630. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, S.; Zhang, K.A.I.; Zhu, J.; Xu, J.; Zhang, C.; Liu, T. Ultrasound-triggered assembly of covalent triazine framework for synthesizing heteroatom-doped carbon nanoflowers boosting metal-free bifunctional electrocatalysis. ACS Appl. Mater. Interfaces 2021, 13, 13328–13337. [Google Scholar] [CrossRef] [PubMed]
- Saha, E.; Karthick, K.; Kundu, S.; Mitra, J. Regulating the heteroatom doping in metallogel-derived Co@dual self-doped carbon onions to maximize electrocatalytic water splitting. J. Mater. Chem. A 2021, 9, 26800–26809. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, F.; Zhang, Y.; Luo, X.; Chen, L.; Shi, Y. ZnS modified N, S dual-doped interconnected porous carbon derived from dye sludge waste as high-efficient ORR/OER catalyst for rechargeable zinc-air battery. J. Colloid Interf. Sci. 2022, 616, 659–667. [Google Scholar] [CrossRef]
- Wang, A.; Zhao, C.; Yu, M.; Wang, W. Trifunctional Co nanoparticle confined in defect-rich nitrogen-doped graphene for rechargeable Zn-air battery with a long lifetime. Appl. Catal. B Environ. 2021, 281, 119514. [Google Scholar] [CrossRef]
- Jiang, T.; Dai, P.; Zhang, W. Fe7S8 nanoparticles encapsulated in porous N, S co-doped carbon as an efficient bifunctional electrocatalyst for Zn-air battery. Micro-Nano Lett. 2020, 15, 495–498. [Google Scholar] [CrossRef]
- Xie, C.; Yan, D.; Li, H.; Du, S.; Chen, W.; Wang, Y.; Zou, Y.; Chen, R.; Wang, S. Defect chemistry in heterogeneous catalysis: Recognition, understanding, and utilization. ACS Catal. 2020, 10, 11082–11098. [Google Scholar] [CrossRef]
- Zhang, Y.; Tao, L.; Xie, C.; Wang, D.; Zou, Y.; Chen, R.; Wang, Y.; Jia, C.; Wang, S. Defect engineering on electrode materials for rechargeable batteries. Adv. Mater. 2020, 32, 1905923. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Kang, B.; Yuan, Y.; Chen, G.; Lee, J.Y. Activating γ-graphyne nanoribbons as bifunctional electrocatalysts toward oxygen reduction and hydrogen evolution reactions by edge termination and nitrogen doping. Chem. Eng. J. 2022, 430, 133126. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, L.; Gao, G.; Chen, H.; Wang, B.; Zhou, J.; Soo, M.T.; Hong, M.; Yan, X.; Qian, G.; et al. A heterostructure coupling of exfoliated Ni-Fe hydroxide nanosheet and defective graphene as a bifunctional electrocatalyst for overall water splitting. Adv. Mater. 2017, 29, 1700017. [Google Scholar] [CrossRef]
- Fan, X.-Z.; Du, X.; Pang, Q.-Q.; Zhang, S.; Liu, Z.-Y.; Yue, X.-Z. In situ construction of bifunctional N-doped carbon-anchored Co nanoparticles for OER and ORR. ACS Appl. Mater. Interfaces 2022, 14, 8549–8556. [Google Scholar] [CrossRef]
- Zhu, Y.; Sokolowski, J.; Song, X.; He, Y.; Mei, Y.; Wu, G. Engineering local coordination environments of atomically dispersed and heteroatom-coordinated single metal site electrocatalysts for clean energy-conversion. Adv. Energy Mater. 2020, 10, 1902844. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, H.; Wang, Y.; Liu, L.; Qin, W.; Liu, S.; Liu, J.; Qin, Y.; Zhang, D.; Chu, A.; et al. Sulfur coordination engineering of molybdenum single-atom for dual-functional oxygen reduction/evolution catalysis. Energy Storage Mater. 2022, 50, 186–195. [Google Scholar] [CrossRef]
- Liu, M.; Li, N.; Cao, S.; Wang, X.; Lu, X.; Kong, L.; Xu, Y.; Bu, X.-H. A “pre-constrained metal twins” strategy to prepare efficient dual-metal-atom catalysts for cooperative oxygen electrocatalysis. Adv. Mater. 2022, 34, 2107421. [Google Scholar] [CrossRef]
- Li, Q.; Lu, L.; Liu, J.; Shi, W.; Cheng, P. Two-dimensional bimetallic coordination polymers as bifunctional evolved electrocatalysts for enhanced oxygen evolution reaction and urea oxidation reaction. J. Energy Chem. 2021, 63, 230–238. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, X.; Yao, S.; Hao, C.; Pan, C.; Xiang, X.; Tian, Z.Q.; Shen, P.K.; Shao, Z.; Jiang, S.P. Boosting electrocatalytic activity of single atom catalysts supported on nitrogen-doped carbon through N coordination environment engineering. Small 2022, 18, 2105329. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Xue, Z.; Yang, J.; Liu, Q.; Xian, J.; Zhong, Y.; Sun, Y.; Zhang, X.; Liu, Q.; Yao, D.; et al. Tailoring the electronic structure of an atomically dispersed zinc electrocatalyst: Coordination environment regulation for high selectivity oxygen reduction. Angew. Chem. Int. Ed. 2022, 61, e202110838. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jia, G.; Cui, X.; Zhao, X.; Zhang, Q.; Gu, L.; Zheng, L.; Li, L.H.; Wu, Q.; Singh, D.J.; et al. Coordination number regulation of molybdenum single-atom nanozyme peroxidase-like specific. Chem 2021, 7, 436–449. [Google Scholar] [CrossRef]
- Zeng, X.; Shui, J.; Liu, X.; Liu, Q.; Li, Y.; Shang, J.; Zheng, L.; Yu, R. Single-atom to single-atom grafting of Pt1 onto Fe-N4 center: Pt1@Fe-N-C multifunctional electrocatalyst with significantly enhanced properties. Adv. Energy Mater. 2018, 8, 1701345. [Google Scholar] [CrossRef]








| Materials | Electrolyte | Overpotential (mV) | Tafel Slope (mV/dec) | Ref. |
|---|---|---|---|---|
| Ni/B, N co–doped carbon nanowires | 1.0 M KOH | 247 | 19 | [88] |
| N–CuCo2O4/N–C | 1.0 M KOH | 260 | 90 | [89] |
| S-FeCo3P/N, P, S tri-doped graphene | 1.0 M KOH | 310 | 75 | [90] |
| N, P, F tri–doped porous carbon | 1.0 M KOH | 330 | 88 | [91] |
| N, O–doped carbon-coated Co–nanoparticles | 1.0 M KOH | 378 | 60 | [92] |
| ZnS/N, S dual–doped porous carbon | 0.1 M KOH | 390 | 117 | [93] |
| Co/N–doped graphene | 0.1 M KOH | 383 | 91 | [94] |
| Fe7S8/N, S co–doped porous carbon | 0.1 M KOH | 450 | 112 | [95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Liu, Y.; Ma, X.; Liu, X.; Zhang, R.; Wang, Y. Metal–Support Interaction of Carbon–Based Electrocatalysts for Oxygen Evolution Reaction. Nanoenergy Adv. 2023, 3, 48-72. https://doi.org/10.3390/nanoenergyadv3010004
Zhang X, Liu Y, Ma X, Liu X, Zhang R, Wang Y. Metal–Support Interaction of Carbon–Based Electrocatalysts for Oxygen Evolution Reaction. Nanoenergy Advances. 2023; 3(1):48-72. https://doi.org/10.3390/nanoenergyadv3010004
Chicago/Turabian StyleZhang, Xiaoyun, Yuxin Liu, Xiaoshuang Ma, Xiaojin Liu, Renyun Zhang, and Yuqiao Wang. 2023. "Metal–Support Interaction of Carbon–Based Electrocatalysts for Oxygen Evolution Reaction" Nanoenergy Advances 3, no. 1: 48-72. https://doi.org/10.3390/nanoenergyadv3010004