A Review of Bifunctional Catalysts for Zinc-Air Batteries
Abstract
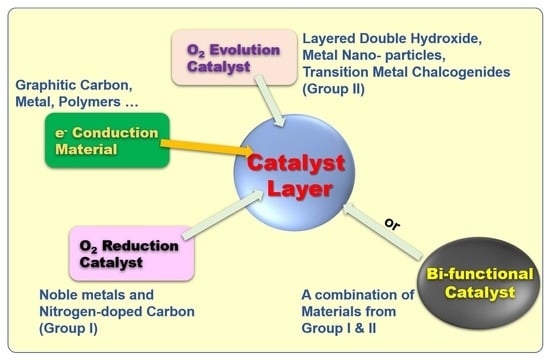
:1. Introduction
2. Noble Metals as Advanced Bifunctional Catalysts in Zn–Air Batteries
3. N-Doped Carbonaceous (NC) Materials as Advanced Bifunctional Catalysts in Zn–Air Batteries
3.1. Metal-Doped NC
3.2. Non-Metal-Doped NC

4. Metallic Nanoparticles as Advanced Bifunctional Catalysts in Zn–Air Batteries
4.1. Single Metallic Particles
4.2. Bimetallic Particles

5. Layered Double Hydroxides as Advanced Bifunctional Catalysts in Zn–Air Batteries

6. Metal Chalcogenides as Advanced Bifunctional Catalysts in Zn–Air Batteries

7. Metal Oxides as Advanced Bifunctional Catalysts in Zn–Air Batteries

| Number Catalyst | E1/2/V | Electrolyte | Catalyst Loadings (mg cm−2) | Ref | |
|---|---|---|---|---|---|
| Noble metal | |||||
| 1 | Fe3Pt/C | 0.91 | 0.1 M KOH | 0.12 | [15] |
| 2 | Fe3Pt/Ni3FeN | 0.93 | 0.1 M KOH | 0.12 | [15] |
| 3 | Co(OH)2/CoPt/N-CN | 0.83 | 0.1 M KOH | 0.102 | [17] |
| 4 | SA-PtCoF | 0.88 | 1 M KOH | 0.42 | [18] |
| 5 | Pt/CoFe2O4 | 0.72 | 0.1 M KOH | 0.283 | [19] |
| 6 | PtFeNC | 0.895 | 0.1 M KOH | 0.255 | [16] |
| 8 | Ag/LMO-NR/RGO | 0.691 | 0.1 M KOH | 0.317 | [22] |
| 9 | Ag-Cu/Ni | 0.691 | 0.1 M KOH | 0.8 | [23] |
| N-doped carbonaceous (NC) materials | |||||
| 10 | NDGs-800 | 0.85 | 0.1 M KOH | 0.204 | [34] |
| 11 | SilkNC/KB | 0.764 | 0.1 M KOH | 0.6 | [35] |
| 12 | GNCNTs-4 | 0.85 | 0.1 M KOH | 0.51 | [37] |
| 13 | NPC@CNF-950 | 0.88 | 0.1 M KOH | 0.1 | [36] |
| 14 | Honeycomb-600 | 0.837 | 0.1 M KOH | 0.3 | [38] |
| 15 | 3D Fe/N-G | 0.852 | 0.1 M KOH | 0.276 | [43] |
| 16 | Fe-MNC | 0.85 | 0.1 M KOH | 0.286 | [44] |
| 17 | 2D Fe-NG | 0.86 | 0.1 M KOH | 0.236 | [45] |
| 18 | GSC-900 | 0.8 | 0.1 M KOH | 0.255 | [49] |
| 19 | hSNCNC | 0.792 | 0.1 M KOH | 0.127 | [52] |
| 20 | DDPCN | 0.87 | 0.1 M KOH | 0.4 | [53] |
| Metallic nanoparticle | |||||
| 21 | Co-N-CNTs | 0.90 | 0.1 M KOH | 0.81 | [60] |
| 22 | CNCF-800 | 0.66 | 0.1 M KOH | 0.25 | [61] |
| 23 | Co@NCNTA-700 | 0.861 | 0.1 M KOH | 1 | [62] |
| 24 | Co@NC-3 | 0.816 | 0.1 M KOH | 0.24 | [63] |
| 25 | Co/N-CNTs@rGO | 0.88 | 0.1 M KOH | [64] | |
| 26 | Co@NC-5 | 0.86 | 0.1 M KOH | 0.5 | [65] |
| 27 | FeCo@NC-750 | 0.8 | 0.1 M KOH | 0.4 | [75] |
| 28 | h-FeCo alloy/N-CNFs | 0.87 | 0.1 M KOH | 0.2 | [76] |
| 29 | CoFe@NCNTs | 0.84 | 0.1 M KOH | 0.8 | [77] |
| 30 | Fe1.2Co@NC/NCNTs | 0.82 | 0.1 M KOH | 0.2 | [78] |
| 31 | Ni3Fe/N-C | 0.81 | 0.1 M KOH | 0.22 | [80] |
| 32 | Ni3Fe/NPG-1 | 0.83 | 0.1 M KOH | 1 | [81] |
| 33 | Fe-enriched-FeNi3/NC | 0.78 | 0.1 M KOH | 0.8 | [82] |
| 34 | Ni3Fe/N-S-CNTs | 0.877 | 1 M KOH | 0.42 | [85] |
| 35 | FeNi/N-CPCF-950 | 0.867 | 0.1 M KOH | 0.6 | [84] |
| 36 | NiFe@NCNTs (Ni0.36Fe0.64) | 0.79 | 1 M KOH | 0.408 | [83] |
| 37 | FeNi3@NC) | 0.86 | 0.1 M KOH | -- | [139] |
| Layered double hydroxide | |||||
| 38 | MCN-LDH-1.5 | 0.826 | 0.1 M KOH | 0.4 | [94] |
| 39 | Co3O4@NiFe-2 | 0.76 | 1 M KOH | 0.4 | [97] |
| 40 | NiCo2S4@NiFe | 0.85 | 0.1 M KOH | -- | [98] |
| 41 | NiMn (Ni3Mn1) | 0.74 | 0.1 M KOH | 0.2 | [99] |
| 42 | Hollow sphere Co2Mn1 | 0.83 | 0.1 M KOH | 0.51 | [101] |
| 43 | CoNi-NS/rGO | 0.85 | 1 M KOH | 0.48 | [102] |
| 44 | CoMn-LDH/NPGA | 0.868 | 0.1 M KOH | 0.255 | [103] |
| Metal chalcogenides | |||||
| 45 | CoSx@PCN/rGO | 0.78 | 0.1 M KOH | 0.408 | [106] |
| 46 | Co/N/S-800 | 0.831 | 0.1 M KOH | 0.1 | [107] |
| 47 | S-Co9−xFexS8@rGO | 0.84 | 0.1 M KOH | 0.5 | [109] |
| 48 | FeCoMoS@NG | 0.83 | 0.1 M KOH | 0.21 | [112] |
| 49 | (Fe,Co)SPPc-900-sp | 0.83 | 0.1 M KOH | 0.28 | [111] |
| 50 | Ni0.75Se | 0.74 | 1 M KOH | [113] | |
| 51 | NiS2/CoS2 | 0.79 | 0.1 M KOH | [116] | |
| 52 | NiS2/CoS2-O NWs | 0.7 | 0.1 M KOH | [117] | |
| 53 | N, P/CoS2@TiO2 | 0.71 | 0.1 M KOH | [118] | |
| Metal oxides | |||||
| 54 | NC-Co3O4-90 | 0.87 | 1 M KOH | [126] | |
| 55 | CoOx/NMC | 0.907 | 1 M KOH | [129] | |
| 56 | NP-Co3O4/CC | 0.9 | 1 M KOH | [130] | |
| 57 | S5.84%-LaCoO3 | 0.7 | 1 M KOH | [137] | |
| 58 | S–CoMn2O4 | 0.76 | 1 M KOH | [138] | |
8. Zinc–Air Battery Performance
| Catalyst | Loading (mg cm−2) | Polarization (∆E/V) | Open Circuit Voltage (V) | Rechargeability (Cycle Number, 1st Charge/Discharge Voltages, Cycle Time/min) | Reference |
|---|---|---|---|---|---|
| RuSn | 20 | 0.7 | 1.36 | 100, 1.9/1.2, 20 | [25] |
| P,S-CNS | 0.45 | 0.8 | 1.51 | >500, 2.02/1.22, 12 | [144] |
| PS-CNFs | 0.20 | 0.81 | 1.49 | 600, 2.2/1.19, 12 | [145] |
| CNT@POF | 1.50 | 0.78 | 1.39 | 200, 1.99/1.21, 20 | [146] |
| Fe(Co)-Nx/C | 1.0 | 0.73 | 1.48 | 2000, 1.97/1.24, 20 | [147] |
| DDPCN | n/a | 0.9 | 1.48 | 360, 2.1/1.2, 5 | [53] |
| CK-800-800 | 4 | 1.21 | 1.51 | 300, 2.41/ 1.2, 10 | [148] |
| Fe–NCNS | 1 | 0.7 | 1.49 | 1000, 1.90/1.2, 10 | [149] |
| 2D Fe-NG | 1 | 0.91 | 1.51 | 480, 2.1/1.19, 10 | [45] |
| Co-N-C | 5 | 0.40 | 1.40 | 72, 1.75/1.35, 20 | [40] |
| FeNx-embedded PNC | 8 | 0.8 | 1.55 | 300, 2.0/1.2, 10 | [48] |
| E-CoSA/N/C | 1 | 0.79 | 1.49 | 1000, 2.0/1.21, 10 | [41] |
| Co–N–C nanobrushes | 1 | 0.84 | 1.50 | 420, 2.05/1.21, 20 | [150] |
| GNCNTs | 1 | 0.8 | 1.48 | 9000, 1.9/1.1, 20 | [37] |
| Co@NC-3 | 0.5 | 0.95 | 1.377 | 450, 2.2/1.25, 10 | [63] |
| Co2Fe1@NC | 2 | 0.75 | 1.45 | 750, 2.0/1.25, 10 | [71] |
| Co@NC | 1 | 0.74 | 1.46 | 300, 1.97/1.23, 10 | [65] |
| FeCo@NC | 1 | 0.7 | 1.38 | 200, 1.9/1.2, 10 | [75] |
| CoFe@NCNTs | 1 | 0.7 | 1.45 | 500, 1.9/1.2, 10 | [77] |
| Ni3Fe/N-S-CNTs | 1 | 0.6 | 1.41 | 500, 1.85/1.25, 60 | [85] |
| Fe1.2Co@NC/NCNTs | 2.0 | 0.74 | 1.43 | 300, 1.98/1.24, 10 | [78] |
| FeCo-NCps | n/a | 0.71 | 1.43 | 1400, 1.9/1.19, 6.7 | [67] |
| Ni3Fe/N-C | 0.5 | 0.95 | 1.5 | 402, 2.05/1.1, 20 | [80] |
| FeCo-N-C-700 | 3 | 0.46 | 1.39 | 240, 1.72/1.26, 10 | [66] |
| NiFe@N-CFs | 1.5 | 0.7 | 1.4 | 300, 1.92/1.26, 40 | [151] |
| FeNi/N-CPCF | 2.0 | 0.77 | 1.478 | 960, 1.99/1.22, 40 | [84] |
| P–FeNi/NC@G | 1.1 | 0.68 | 1.53 | 144, 1.9/1.18, 10 | [152] |
| NiFe@NCNTs | n/a | 0.6 | 1.48 | 2400, 1.9/1.3, 5 | [83] |
| CoFe@NC/KB-800 | 0.5 | 0.5 | 1.44 | 600, 1.8/1.3, 10 | [69] |
| Co–Nx/C | 1.0 | 0.68 | 1.42 | 120, 1.93/1.25, 5 | [153] |
| Ni/NiO nanosheets | 2 | 0.73 | 1.47 | 720, 1.99/1.26, 10 | [154] |
| Co@Co4N/MnO-NC | 2.5~3.0 | 0.87 | 1.47 | 2800, 2.14/1.27, 10 | [155] |
| FexNiyN@C/NC | 2 | 0.9 | 1.25 | 400, 1.95/1.05, 60 | [156] |
| NiFe LDH-CoPc/CNTs | n/a | 0.67 | 1.40 | 550, 1.90/1.23, 120 | [93] |
| NiCo2O4@NiMn LDH | 2.0 | 0.92 | 1.40 | 500, 2.1/1.18, 10 | [100] |
| Co3O4@NiFe LDHs | n/a | 0.78 | 1.5 | 1250, 1.98/1.2, 10 | [97] |
| MCN-LDH | 3 | 1.0 | 1.4 | 430, 2/1, 16.7 | [94] |
| MnOx-NiFe LDH/Ni | n/a | 0.7 | 1.4 | 660, 1.9/1.2, 10 | [92] |
| CoNi-NS/rGO | 1 | 0.81 | 1.37 | 350, 2.0/1.19, 1.67 | [102] |
| NiCo2O4@FeNi LDH | n/a | 0.65 | 1.40 | 90, 1.9/1.25, 60 | [96] |
| NiFe LDH | 1.20 | 0.85 | n/a | 600, 1.95/1.10, 10 | [157] |
| CoMn-LDH/NPGA | n/a | 0.83 | 1.514 | 432, 2.04/1.21, 10 | [103] |
| Co9S8/S-rGO | 10 | 0.65 | 1.42 | 150, 1.95/1.3, 20 | [158] |
| S-GNS/NiCo2S4 | n/a | 0.7 | 1.31 | 150, 1.9/1.2, 40 | [159] |
| Co/S/N-800 | 1.0 | 0.65 | 1.54 | 72, 1.97/1.32, 40 | [107] |
| CuCo2S4NSs@N-CNFs | n/a | 0.9 | 1.46 | 300, 2.1/1.2, 20 | [160] |
| FeCoMoS@NG | 1 | 0.7 | 1.44 | 420, 1.95/1.25, 10 | [112] |
| Co9S8/GN | 2 | 0.55 | 1.41 | 30, 1.8/1.25, 10 | [108] |
| Ni1−xMoxOSe | 1.5 | 0.73 | 1.429 | 300, 1.95/1.22, 60 | [161] |
| FeS2–CoS2/NCFs | 1.2 | 0.88 | 1.46 | 1500, 2.08/1.2, 10 | [162] |
| Fe/N/C@BMZIF | 1 | 0.82 | 1.48 | 100, 1.95/1.13, 10 | [163] |
| Fe-N/C | 2 | 1.0 | 1.525 | 50, 2.1/1.1, 10 | [164] |
| Ni2.25Co0.75N/NrGO | 2.85 | 0.75 | 1.43 | 500, 1.97/1.22, 20 | [165] |
| MnxFe3-xC/NC | 2 | 1.1 | 1.5 | 1000, 2.2/ 1.1, 20 | [166] |
| Fe2Ni2N/Co@NCNT | 2 | 0.75 | 1.3 | 850, 1.9/1.15, 60 | [143] |
| CuSA@HNCNx | 0.5 | 0.65 | 1.51 | 1800, 1.95/1.3, 10 | [167] |
| Co@WC1−x/NCNTs | 1.0 | 0.9 | 1.452 | 200, 2.0/1.1, 20 | [168] |
| Zn-Ni3FeN/NG | 1 | 0.8 | 1.60 | 540, 1.95/1.15, 20 | [141] |
| FeNxC | 0.5 | 0.65 | 1.41 | 600, 1.85/1.2, 10 | [169] |
| TCoNCNTs + NiFe-LDH | 2 + 1 | 0.7 | 1.39 | 100, 1.95/1.25, 50 | [170] |
| NCO-250 | 1 | 0.77 | 1.51 | 225, 2.0/1.23, 20 | [171] |
| Pd-La0.7Sr0.3CoO3-δ | 1 | 1.2 | 1.5 | 60, 2.2/ 1.0, 60 | [172] |
| NP-Co3O4/CC | 0.8 | 0.8 | 1.576 | 1200, 1.9/1.1, 20 | [130] |
| MnO2-Fe2O3/CNT | 1.0 | 0.69 | 1.51 | 360, 1.95/1.26, 10 | [173] |
| S–CoMn2O4 | 1.0 | 0.8 | 1.52 | 180, 2.0/1.2, 20 | [138] |
9. Summary and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dunn, H.B.; Tarascon, J.-M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-S.; Kim, S.T.; Cao, R.; Choi, N.-S.; Liu, M.; Lee, K.; Cho, J. Metal-Air Batteries with High Energy Density: Li-Air versus Zn-Air. Adv. Energy Mater. 2011, 1, 34–50. [Google Scholar] [CrossRef]
- Lim, M.B.; Lambert, T.N. Rechargeable Zinc Batteries for Grid Storage, 2020 U.S. DOE Energy Storage Handbook; Sandia National Laboratory: Albuquerque, NM, USA, 2020. Available online: http://www.sandia.gov/ (accessed on 30 January 2023).
- Fu, J.; Cano, Z.; Park, M.; Yu, A.; Fowler, M.; Chen, Z. Electrically Rechargeable Zinc-Air Batteries: Progress, Challenges, and Perspectives. Adv. Mater. 2017, 29, 1604685. [Google Scholar] [CrossRef] [PubMed]
- Akhil, G.A.A.; Currier, A.B.; Kaun, B.C.; Rastler, D.M.; Chen, S.B.; Cotter, A.L.; Bradshaw, D.T.; Gauntlett, W.D. DOE/EPRI Electricity Storage Handbook in Collaboration with NRECA; Sandia National Laboratory: Albuquerque, NM, USA, 2015. Available online: http://www.osti.gov/bridge (accessed on 30 January 2023).
- Zhang, J.; Vukmirovic, M.; Xu, Y.; Mavrikakis, M.; Adzic, R. Controlling the Catalytic Activity of Platinum-Monolayer Electrocatalysts for Oxygen Reduction with Different Substrates. Angew. Chem. 2005, 117, 2170–2173. [Google Scholar] [CrossRef]
- Borodzinski, G. Relation between the surface states of oxide films at Rh electrodes and kinetics of the oxygen evolution reaction. J. Chem. Soc. Faraday Trans. 1994, 90, 3669–3675. [Google Scholar]
- Antolini, E. Iridium As Catalyst and Cocatalyst for Oxygen Evolution/Reduction in Acidic Polymer Electrolyte Membrane Electrolyzers and Fuel Cells. ACS Catal. 2014, 4, 1426–1440. [Google Scholar] [CrossRef]
- Da Silva, M.G.C.; Ticianelli, E.A. Activity and Stability of Pt/IrO2 Bifunctional Materials as Catalysts for the Oxygen Evolution/Reduction Reactions. ACS Catal. 2018, 8, 2081–2092. [Google Scholar] [CrossRef]
- Ioroi, N.T.; Yasuda, K.; Yamamoto, Y.; Takenaka, H. Iridium Oxide/Platinum Electrocatalysts for Unitized Regenerative Polymer Electrolyte Fuel Cells. J. Electrochem. Soc. 2000, 147, 2018–2022. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.; Wan, N.; Mao, Z. Deposited RuO2–IrO2/Pt electrocatalyst for the regenerative fuel cell. Int. J. Hydrogen Energy 2007, 32, 400–404. [Google Scholar] [CrossRef]
- Spendelow, J.; Wieckowski, A. Electrocatalysis of oxygen reduction and small alcohol oxidation in alkaline media. Phys. Chem. Chem. Phys. PCCP 2007, 9, 2654–2675. [Google Scholar] [CrossRef]
- Bak, J.; Kim, H.; Lee, S.; Kim, M.; Kim, E.-J.; Roh, J.; Shin, J.; Choi, C.; Cho, E. Boosting the Role of Ir in Mitigating Corrosion of Carbon Support by Alloying with Pt. ACS Catal. 2020, 10, 12300–12309. [Google Scholar] [CrossRef]
- Islam, J.; Kim, S.-K.; Kim, K.-H.; Lee, E.; Park, G.-G. Enhanced durability of Pt/C catalyst by coating carbon black with silica for oxygen reduction reaction. Int. J. Hydrogen Energy 2021, 46, 1133–1143. [Google Scholar] [CrossRef]
- Cui, Z.; Fu, G.; Li, Y.; Goodenough, J. Ni3FeN-Supported Fe3Pt Intermetallic Nanoalloy as a High-Performance Bifunctional Catalyst for Metal-Air Batteries. Angew. Chem. 2017, 56, 9901–9905. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Ye, S.; Tang, J.; Zhu, Y.; Wu, D.; Gu, M.; Pan, H.; Xu, B. Engineering Pt and Fe dual-metal single atoms anchored on nitrogen-doped carbon with high activity and durability towards oxygen reduction reaction for zinc-air battery. Appl. Catal. B: Environ. 2021, 286, 119891. [Google Scholar] [CrossRef]
- Wang, K.; Wu, W.; Tang, Z.; Li, L.; Chen, S.; Bedford, N. Hierarchically Structured Co(OH)2/CoPt/N-CN Air Cathodes for Rechargeable Zinc-Air Batteries. ACS Appl. Mater. Interfaces 2019, 11, 4983–4994. [Google Scholar] [CrossRef]
- Li, Z.; Niu, W.; Yang, Z.; Zaman, N.; Samarakoon, W.; Wang, M.; Kara, A.; Lucero, M.; Vyas, M.; Cao, H.; et al. Stabilizing atomic Pt with trapped interstitial F in alloyed PtCo nanosheets for high-performance zinc-air batteries. Energy Environ. Sci. 2020, 13, 884–895. [Google Scholar] [CrossRef]
- Su, Z.; Liu, X.; Hao, S.; Li, Z.; Yang, B.; Hou, Y.; Lei, L.; Zhang, X. Pt/CoFe2O4-C hollow ball as efficient bifunctional electrocatalyst for Zn-air batteries. Catal. Today 2021, 368, 204–210. [Google Scholar] [CrossRef]
- Shinagawa, T.; Garcia-Esparza, A.; Takanabe, K. Insight on Tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion. Sci. Rep. 2015, 5, 13801. [Google Scholar] [CrossRef]
- Liang, Y.; Lei, H.; Wang, S.; Wang, Z.; Mai, W. Pt/Zn heterostructure as efficient air-electrocatalyst for long-life neutral Zn-air batteries. Sci. China Mater. 2021, 64, 1868–1875. [Google Scholar] [CrossRef]
- Hu, J.; Liu, Q.; Shi, L.; Shi, Z.; Huang, H. Silver decorated LaMnO3 nanorod/graphene composite electrocatalysts as reversible metal-air battery electrodes. Appl. Surf. Sci. 2017, 402, 61–69. [Google Scholar] [CrossRef]
- Jin, Y.; Chen, F. Facile preparation of Ag-Cu bifunctional electrocatalysts for zinc-air batteries. Electrochim. Acta 2015, 158, 437–445. [Google Scholar] [CrossRef]
- Wu, X.; Chen, F.; Zhang, N.; Qaseem, A.; Johnston, R. A silver–copper metallic glass electrocatalyst with high activity and stability comparable to Pt/C for zinc–air batteries. J. Mater. Chem. A 2016, 4, 3527–3537. [Google Scholar] [CrossRef]
- You, T.; Hu, C. Designing Binary Ru-Sn Oxides with Optimized Performances for the Air Electrode of Rechargeable Zinc-Air Batteries. ACS Appl. Mater. Interfaces 2018, 10, 10064–10075. [Google Scholar] [CrossRef] [PubMed]
- Gong, F.K.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-Doped Carbon Nanotube Arrays with High Electrocatalytic Activity for Oxygen Reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.; Wang, Q.; Zhang, L.; Liang, H.-W.; Chen, P.; Yu, S.-H. N-, P- and Fe-tridoped nanoporous carbon derived from plant biomass: An excellent oxygen reduction electrocatalyst for zinc–air batteries. J. Mater. Chem. A 2016, 4, 8602–8609. [Google Scholar] [CrossRef]
- Qian, Y.; Hu, Z.; Ge, X.; Yang, S.; Peng, Y.; Kang, Z.; Liu, Z.; Lee, J.; Zhao, D. A metal-free ORR/OER bifunctional electrocatalyst derived from metal-organic frameworks for rechargeable Zn-Air batteries. Carbon 2017, 111, 641–650. [Google Scholar] [CrossRef]
- Jin, H.; Zhou, H.; Li, W.; Wang, Z.; Yang, J.; Xiong, Y.; He, D.; Chen, L.; Mu, S. In situ derived Fe/N/S-codoped carbon nanotubes from ZIF-8 crystals as efficient electrocatalysts for the oxygen reduction reaction and zinc–air batteries. J. Mater. Chem. A 2018, 6, 20093–20099. [Google Scholar] [CrossRef]
- Wang, H.-F.; Tang, C.; Wang, B.; Li, B.-Q.; Cui, X.; Zhang, Q. Defect-rich carbon fiber electrocatalysts with porous graphene skin for flexible solid-state zinc–air batteries. Energy Storage Mater. 2018, 15, 124–130. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, Y.; Wang, Q.; Liao, Z.; Zhang, N.; Guo, Y.; Xiang, Z. Hierarchically porous metal-free carbon with record high mass activity for oxygen reduction and Zn-air batteries. J. Mater. Chem. A 2019, 7, 9831–9836. [Google Scholar] [CrossRef]
- Cai, X.; Xia, B.; Franklin, J.; Li, B.; Wang, X.; Wang, Z.; Chen, L.; Lin, J.; Lai, L.; Shen, Z. Free-standing vertically-aligned nitrogen-doped carbon nanotube arrays/graphene as air-breathing electrodes for rechargeable zinc–air batteries. J. Mater. Chem. A 2017, 5, 2488–2495. [Google Scholar] [CrossRef]
- Kim, H.; Lee, K.; Woo, S.; Jung, Y. On the mechanism of enhanced oxygen reduction reaction in nitrogen-doped graphene nanoribbons. Phys. Chem. Chem. Phys. PCCP 2011, 13, 17505–17510. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Lei, Y.; Wang, Y.; Wang, Y.; Li, Y.; Wang, S. Pyridinic-N-Dominated Doped Defective Graphene as a Superior Oxygen Electrocatalyst for Ultrahigh-Energy-Density Zn–Air Batteries. ACS Energy Lett. 2018, 3, 1183–1191. [Google Scholar] [CrossRef]
- Wang, C.; Xie, N.-H.; Zhang, Y.; Huang, Z.; Xia, K.; Wang, H.; Guo, S.; Xu, B.-Q.; Zhang, Y. Silk-Derived Highly Active Oxygen Electrocatalysts for Flexible and Rechargeable Zn–Air Batteries. Chem. Mater. 2019, 31, 1023–1029. [Google Scholar] [CrossRef]
- Li, Y.; Chen, M.; Chu, M.; Wang, X.; Wang, Y.; Lin, X.; Cao, X. Mono-Doped Carbon Nanofiber Aerogel as a High-Performance Electrode Material for Rechargeable Zinc-Air Batteries. ChemElectroChem 2021, 8, 829–838. [Google Scholar] [CrossRef]
- Xu, Y.; Deng, P.; Chen, G.; Chen, J.; Yan, Y.; Qi, K.; Liu, H.; Xia, B. 2D Nitrogen-Doped Carbon Nanotubes/Graphene Hybrid as Bifunctional Oxygen Electrocatalyst for Long-Life Rechargeable Zn–Air Batteries. Adv. Funct. Mater. 2019, 30, 1906081. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, G.; Deng, Y.; Liu, G.; Ren, D.; Zhang, Z.; Zhu, J.; Gao, R.; Jiang, Y.; Luo, D.; et al. Deep-Breathing Honeycomb-like Co-Nx-C Nanopolyhedron Bifunctional Oxygen Electrocatalysts for Rechargeable Zn-Air Batteries. iScience 2020, 23, 101404. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Wang, B.; Wang, H.; Zhang, Q. Defect Engineering toward Atomic Co-Nx-C in Hierarchical Graphene for Rechargeable Flexible Solid Zn-Air Batteries. Adv. Mater. 2017, 29, 1703185. [Google Scholar] [CrossRef]
- Li, J.-C.; Wu, X.-T.; Chen, L.-J.; Li, N.; Liu, Z.-Q. Bifunctional MOF-derived Co-N-doped carbon electrocatalysts for high-performance zinc-air batteries and MFCs. Energy 2018, 156, 95–102. [Google Scholar] [CrossRef]
- Liu, S.; Ji, H.; Wang, M.; Sun, H.; Liu, J.; Yan, C.; Qian, T. Atomic Metal Vacancy Modulation of Single-Atom Dispersed Co/N/C for Highly Efficient and Stable Air Cathode. ACS Appl. Mater. Interfaces 2020, 12, 15298–15304. [Google Scholar] [CrossRef]
- Chen, J.; Li, W.; Xu, B. Nitrogen-rich Fe-N-C materials derived from polyacrylonitrile as highly active and durable catalysts for the oxygen reduction reaction in both acidic and alkaline electrolytes. J. Colloid. Interface Sci. 2017, 502, 44–51. [Google Scholar] [CrossRef]
- Wang, C.; Li, Z.; Wang, L.; Niu, X.; Wang, S. Facile Synthesis of 3D Fe/N Codoped Mesoporous Graphene as Efficient Bifunctional Oxygen Electrocatalysts for Rechargeable Zn–Air Batteries. ACS Sustain. Chem. Eng. 2019, 7, 13873–13885. [Google Scholar] [CrossRef]
- Luo, X.; Liu, Z.; Ma, Y.; Nan, Y.; Gu, Y.; Li, S.; Zhou, Q.; Mo, J. Biomass derived Fe,N-doped carbon material as bifunctional electrocatalysts for rechargeable Zn-air batteries. J. Alloys Compd. 2021, 888, 161464. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Y.; Li, Z.; Wang, L.; Niu, X.; Sun, P. Novel space-confinement synthesis of two-dimensional Fe, N-codoped graphene bifunctional oxygen electrocatalyst for rechargeable air-cathode. Chem. Eng. J. 2021, 411, 128492. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, Y.; Fan, C.; Wang, J.; Du, F.; Jiang, L. Three-Dimensional Porous Fe–N–C Derived from Iron-Citrate-Functionalized Melamine Foam as a Highly Active Oxygen Reduction Catalyst for Zn–Air Batteries. Energy Technol. 2020, 8, 2000149. [Google Scholar] [CrossRef]
- Du, C.; Gao, Y.; Wang, J.; Chen, W. A new strategy for engineering a hierarchical porous carbon-anchored Fe single-atom electrocatalyst and the insights into its bifunctional catalysis for flexible rechargeable Zn–air batteries. J. Mater. Chem. A 2020, 8, 9981–9990. [Google Scholar] [CrossRef]
- Ma, L.; Chen, S.; Pei, Z.; Huang, Y.; Liang, G.; Mo, F.; Yang, Q.; Su, J.; Gao, Y.; Zapien, J.; et al. Single-Site Active Iron-Based Bifunctional Oxygen Catalyst for a Compressible and Rechargeable Zinc-Air Battery. ACS Nano 2018, 12, 1949–1958. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Wang, K.; Qiu, Y.; Liu, X.; Cao, C.; Feng, Y.; Hu, P. Nitrogen and sulfur co-doped porous carbon derived from bio-waste as a promising electrocatalyst for zinc-air battery. Energy 2018, 143, 43–55. [Google Scholar] [CrossRef]
- Zhu, J.; Li, W.; Li, S.; Zhang, J.; Zhou, H.; Zhang, C.; Zhang, J.; Mu, S. Defective N/S-Codoped 3D Cheese-Like Porous Carbon Nanomaterial toward Efficient Oxygen Reduction and Zn-Air Batteries. Small 2018, 14, e1800563. [Google Scholar] [CrossRef]
- Kim, M.-J.; Park, J.; Kim, S.; Lim, M.; Jin, A.; Kim, O.-H.; Kim, M.; Lee, K.-S.; Kim, J.; Kim, S.-S.; et al. Biomass-Derived Air Cathode Materials: Pore-Controlled S,N-Co-doped Carbon for Fuel Cells and Metal–Air Batteries. ACS Catal. 2019, 9, 3389–3398. [Google Scholar] [CrossRef]
- Fan, H.; Wang, Y.; Gao, F.; Yang, L.; Liu, M.; Du, X.; Wang, P.; Yang, L.; Wu, Q.; Wang, X.; et al. Hierarchical sulfur and nitrogen co-doped carbon nanocages as efficient bifunctional oxygen electrocatalysts for rechargeable Zn-air battery. J. Energy Chem. 2019, 34, 64–71. [Google Scholar] [CrossRef]
- Najam, T.; Shah, S.A.; Ali, H.; Song, Z.; Sun, H.; Peng, Z.; Cai, X. A metal free electrocatalyst for high-performance zinc-air battery applications with good resistance towards poisoning species. Carbon 2020, 164, 12–18. [Google Scholar] [CrossRef]
- Chen, S.; Cui, M.; Yin, Z.; Zeng, Q.; Cao, Z.; Xiong, J.; Mi, L.; Li, Y. Confined Synthesis of N, P Co–Doped 3D Hierarchical Carbons as High-Efficiency Oxygen Reduction Reaction Catalysts for Zn–Air Battery. ChemElectroChem 2020, 7, 4131–4135. [Google Scholar] [CrossRef]
- Li, Y.; Gong, M.; Liang, Y.; Feng, J.; Kim, J.; Wang, H.; Hong, G.; Zhang, B.; Dai, H. Advanced zinc-air batteries based on high-performance hybrid electrocatalysts. Nat. Commun. 2013, 4, 1805. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dai, H. Recent advances in zinc-air batteries. Chem. Soc. Rev. 2014, 43, 5257–5275. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-F.; Tang, C.; Zhang, Q. A Review of Precious-Metal-Free Bifunctional Oxygen Electrocatalysts: Rational Design and Applications in Zn−Air Batteries. Adv. Funct. Mater. 2018, 28, 1803329. [Google Scholar] [CrossRef]
- Han, C.; Li, W.; Liu, H.-K.; Dou, S.; Wang, J. Design strategies for developing non-precious metal based bi-functional catalysts for alkaline electrolyte based zinc–air batteries. Mater. Horiz. 2019, 6, 1812–1827. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, J.; Li, Z.; Bu, X. Recent Progress on NiFe-Based Electrocatalysts for the Oxygen Evolution Reaction. Small 2020, 16, e2003916. [Google Scholar] [CrossRef]
- Wang, T.; Kou, Z.; Mu, S.; Liu, J.; He, D.; Amiinu, I.; Meng, W.; Zhou, K.; Luo, Z.; Chaemchuen, S.; et al. 2D Dual-Metal Zeolitic-Imidazolate-Framework-(ZIF)-Derived Bifunctional Air Electrodes with Ultrahigh Electrochemical Properties for Rechargeable Zinc-Air Batteries. Adv. Funct. Mater. 2018, 28, 1705048. [Google Scholar] [CrossRef]
- Li, H.; An, M.; Zhao, Y.; Pi, S.; Li, C.; Sun, W.; Wang, H.-G. Co nanoparticles encapsulated in N-doped carbon nanofibers as bifunctional catalysts for rechargeable Zn-air battery. Appl. Surf. Sci. 2019, 478, 560–566. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Yan, F.; Zhu, C.; Geng, B.; Chen, Y.; Chou, S. Cobalt-Encapsulated Nitrogen-Doped Carbon Nanotube Arrays for Flexible Zinc–Air Batteries. Small Methods 2019, 4, 1900571. [Google Scholar] [CrossRef]
- Liu, Y.; Ji, D.; Li, M.; Xiao, J.; Dong, P.; Zhang, C.; Han, L.; Zhang, Y. Facile synthesis of cobalt nanoparticles encapsulated in nitrogen-doped carbon nanotubes for use as a highly efficient bifunctional catalyst in rechargeable Zn-Air batteries. J. Alloys Compd. 2020, 842, 155791. [Google Scholar] [CrossRef]
- Shen, M.; Gao, K.; Duan, C.; Hu, W.; Ding, S.; Yang, G.; Ni, Y. Coordination-driven hierarchically structured composites with N-CNTs-grafted graphene-confined ultra-small Co nanoparticles as effective oxygen electrocatalyst in rechargeable Zn-air battery. Ceram. Int. 2021, 47, 30091–30098. [Google Scholar] [CrossRef]
- Tan, M.; Xiao, Y.; Xi, W.; Lin, X.; Gao, B.; Chen, Y.; Zheng, Y.; Lin, B. Cobalt-nanoparticle impregnated nitrogen-doped porous carbon derived from Schiff-base polymer as excellent bifunctional oxygen electrocatalysts for rechargeable zinc-air batteries. J. Power Sources 2021, 490, 229570. [Google Scholar] [CrossRef]
- Duan, X.; Ren, S.; Pan, N.; Zhang, M.; Zheng, H. MOF-derived Fe,Co@N–C bifunctional oxygen electrocatalysts for Zn–air batteries. J. Mater. Chem. A 2020, 8, 9355–9363. [Google Scholar] [CrossRef]
- Liu, J.; He, T.; Wang, Q.; Zhou, Z.; Zhang, Y.; Wu, H.; Li, Q.; Zheng, J.; Sun, Z.; Lei, Y.; et al. Confining ultrasmall bimetallic alloys in porous N–carbon for use as scalable and sustainable electrocatalysts for rechargeable Zn–air batteries. J. Mater. Chem. A 2019, 7, 12451–12456. [Google Scholar] [CrossRef]
- Fu, G.; Liu, Y.; Chen, Y.; Tang, Y.; Goodenough, J.; Lee, J. Robust N-doped carbon aerogels strongly coupled with iron-cobalt particles as efficient bifunctional catalysts for rechargeable Zn-air batteries. Nanoscale 2018, 10, 19937–19944. [Google Scholar] [CrossRef]
- Ren, S.; Duan, X.; Ge, F.; Chen, Z.; Yang, Q.; Zhang, M.; Zheng, H. Novel MOF-derived hollow CoFe alloy coupled with N-doped Ketjen Black as boosted bifunctional oxygen catalysts for Zn–air batteries. Chem. Eng. J. 2022, 427, 131614. [Google Scholar] [CrossRef]
- Hao, X.; Jiang, Z.; Zhang, B.; Tian, X.; Song, C.; Wang, L.; Maiyalagan, T.; Hao, X.; Jiang, Z.J. N-Doped Carbon Nanotubes Derived from Graphene Oxide with Embedment of FeCo Nanoparticles as Bifunctional Air Electrode for Rechargeable Liquid and Flexible All-Solid-State Zinc-Air Batteries. Adv. Sci. 2021, 8, 2004572. [Google Scholar] [CrossRef]
- Tang, T.; Jiang, W.; Liu, X.; Deng, J.; Niu, S.; Wang, B.; Jin, S.; Zhang, Q.; Gu, L.; Hu, J.; et al. Metastable Rock Salt Oxide-Mediated Synthesis of High-Density Dual-Protected M@NC for Long-Life Rechargeable Zinc-Air Batteries with Record Power Density. J. Am. Chem. Soc. 2020, 142, 7116–7127. [Google Scholar] [CrossRef]
- Wang, M.; Liu, Y.; Zhang, K.; Yu, F.; Qin, F.; Fang, J.; Lai, Y.; Li, J. Metal coordination enhanced Ni–Co@N-doped porous carbon core–shell microsphere bi-functional electrocatalyst and its application in rechargeable zinc/air batteries. RSC Adv. 2016, 6, 83386–83392. [Google Scholar] [CrossRef]
- Deng, Z.; Yi, Q.; Li, G.; Chen, Y.; Yang, X.; Nie, H. NiCo-doped C-N nano-composites for cathodic catalysts of Zn-air batteries in neutral media. Electrochim. Acta 2018, 279, 1–9. [Google Scholar] [CrossRef]
- Asokan, A.; Lee, H.; Gwon, O.; Kim, J.; Kwon, O.; Kim, G. Insights Into the Effect of Nickel Doping on ZIF-Derived Oxygen Reduction Catalysts for Zinc−Air Batteries. ChemElectroChem 2019, 6, 1213–1224. [Google Scholar] [CrossRef]
- Cai, P.; Ci, S.; Zhang, E.; Shao, P.; Cao, C.; Wen, Z. FeCo Alloy Nanoparticles Confined in Carbon Layers as High-activity and Robust Cathode Catalyst for Zn-Air Battery. Electrochim. Acta 2016, 220, 354–362. [Google Scholar] [CrossRef]
- Ma, Y.; Zang, W.; Sumboja, A.; Mao, L.; Liu, X.; Tan, M.; Pennycook, S.; Kou, Z.; Liu, Z.; Li, X.; et al. Hollow structure engineering of FeCo alloy nanoparticles electrospun in nitrogen-doped carbon enables high performance flexible all-solid-state zinc–air batteries. Sustain. Energy Fuels 2020, 4, 1747–1753. [Google Scholar] [CrossRef]
- Cai, P.; Hong, Y.; Ci, S.; Wen, Z. In situ integration of CoFe alloy nanoparticles with nitrogen-doped carbon nanotubes as advanced bifunctional cathode catalysts for Zn-air batteries. Nanoscale 2016, 8, 20048–20055. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, W.; Pan, H.; Cao, Y.; Jiang, Z.; Tian, X.; Hao, X.; Maiyalagan, T.; Jiang, Z.-J. FeCo Alloy Nanoparticles Coated by an Ultrathin N-Doped Carbon Layer and Encapsulated in Carbon Nanotubes as a Highly Efficient Bifunctional Air Electrode for Rechargeable Zn-Air Batteries. ACS Sustain. Chem. Eng. 2019, 7, 8530–8541. [Google Scholar] [CrossRef]
- Corrigan, D. The Catalysis of the Oxygen Evolution Reaction by Iron Impurities in Thin Film Nickel Oxide Electrodes. J. Electrochem. Soc. 1987, 134, 377–384. [Google Scholar] [CrossRef]
- Sun, P.; Zuo, Z.; He, M.; Yang, L.; Zhang, D.; Huang, N.; Chen, Z.; Sun, Y.; Sun, X. N-doped carbon sheets loaded with well-dispersed Ni3Fe nanoparticles as bifunctional oxygen electrode for rechargeable Zn-air battery. J. Electroanal. Chem. 2019, 851, 113418. [Google Scholar] [CrossRef]
- Hao, X.; Jiang, Z.; Tian, X.; Song, C.; Chen, S.; Hao, X.; Jiang, Z.-J.; Ultrasmall, S. Homogeneously Distributed Ni3Fe Alloy Nanoparticles on N-Doped Porous Graphene as a Bifunctional Electrocatalyst for Rechargeable Flexible Solid Zinc-Air Batteries. ACS Appl. Energy Mater. 2020, 3, 12148–12161. [Google Scholar] [CrossRef]
- Chen, K.; Kim, S.; Rajendiran, R.; Prabakar, K.; Li, G.; Shi, Z.; Jeong, C.; Kang, J.; Li, O. Enhancing ORR/OER active sites through lattice distortion of Fe-enriched FeNi3 intermetallic nanoparticles doped N-doped carbon for high-performance rechargeable Zn-air battery. J. Colloid Interface Sci. 2021, 582, 977–990. [Google Scholar] [CrossRef]
- Jiang, M.; Tan, Z.; Cao, M. A robust bifunctional electrocatalyst for rechargeable zinc-air batteries: NiFe nanoparticles encapsulated in nitrogen-doped carbon nanotubes. Int. J. Hydrogen Energy 2021, 46, 15507–15516. [Google Scholar] [CrossRef]
- Wang, Z.; Ang, J.; Liu, J.; Ma, X.; Kong, J.; Zhang, Y.; Yan, T.; Lu, X. FeNi alloys encapsulated in N-doped CNTs-tangled porous carbon fibers as highly efficient and durable bifunctional oxygen electrocatalyst for rechargeable zinc-air battery. Appl. Catal. B Environ. 2020, 263, 118344. [Google Scholar] [CrossRef]
- Lai, C.; Wang, J.; Lei, W.; Xuan, C.; Xiao, W.; Zhao, T.; Huang, T.; Chen, L.; Zhu, Y.; Wang, D. Restricting Growth of Ni3Fe Nanoparticles on Heteroatom-Doped Carbon Nanotube/Graphene Nanosheets as Air-Electrode Electrocatalyst for Zn-Air Battery. ACS Appl. Mater. Interfaces 2018, 10, 38093–38100. [Google Scholar] [CrossRef] [PubMed]
- Bukhtiyarova, M. A review on effect of synthesis conditions on the formation of layered double hydroxides. J. Solid State Chem. 2019, 269, 494–506. [Google Scholar] [CrossRef]
- Yang, Z.-z.; Zhang, C.; Zeng, G.-M.; Tan, X.-F.; Huang, D.-L.; Zhou, J.-W.; Fang, Q.-Z.; Yang, K.-H.; Wang, H.; Wei, J.; et al. State-of-the-art progress in the rational design of layered double hydroxide based photocatalysts for photocatalytic and photoelectrochemical H2/O2 production. Coord. Chem. Rev. 2021, 446, 214103. [Google Scholar] [CrossRef]
- Gao, R.; Zhu, J.; Yan, D. Transition metal-based layered double hydroxides for photo(electro)chemical water splitting: A mini review. Nanoscale 2021, 13, 13593–13603. [Google Scholar] [CrossRef]
- Yi, H.; Liu, S.; Lai, C.; Zeng, G.; Li, M.; Liu, X.; Li, B.; Huo, X.; Qin, L.; Li, L.; et al. Recent Advance of Transition-Metal-Based Layered Double Hydroxide Nanosheets: Synthesis, Properties, Modification, and Electrocatalytic Applications. Adv. Energy Mater. 2021, 11, 2002863. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Z.; Zhu, S.; Feng, L.; Xing, W. Ni-based layered double hydroxide catalysts for oxygen evolution reaction. Mater. Today Phys. 2020, 16, 100292. [Google Scholar] [CrossRef]
- Wang, P.; Lin, Y.; Wan, L.; Wang, B. Construction of a Janus MnO2-NiFe Electrode via Selective Electrodeposition Strategy as a High-Performance Bifunctional Electrocatalyst for Rechargeable Zinc-Air Batteries. ACS Appl. Mater. Interfaces 2019, 11, 37701–37707. [Google Scholar] [CrossRef]
- Wan, L.; Wang, P.; Lin, Y.; Wang, B. Janus-Typed Integrated Bifunctional Air Electrode with MnOx-NiFe LDH/Ni Foam for Rechargeable Zinc-Air Batteries. J. Electrochem. Soc. 2019, 166, A3409–A3415. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, D.; Zhou, L.; Yu, H.; Huang, B. NiFe LDH-CoPc/CNTs as novel bifunctional electrocatalyst complex for zinc–air battery. Ionics 2017, 24, 1709–1714. [Google Scholar] [CrossRef]
- Qian, Y.; An, T.; Sarnello, E.; Liu, Z.; Li, T.; Zhao, D. Janus Electrocatalysts Containing MOF-Derived Carbon Networks and NiFe-LDH Nanoplates for Rechargeable Zinc–Air Batteries. ACS Appl. Energy Mater. 2019, 2, 1784–1792. [Google Scholar] [CrossRef]
- Chen, D.; Chen, X.; Cui, Z.; Li, G.; Han, B.; Zhang, Q.; Sui, J.; Dong, H.; Yu, J.; Yu, L.; et al. Dual-active-site hierarchical architecture containing NiFe-LDH and ZIF-derived carbon-based framework composite as efficient bifunctional oxygen electrocatalysts for durable rechargeable Zn-air batteries. Chem. Eng. J. 2020, 399, 125718. [Google Scholar] [CrossRef]
- Wan, L.; Zhao, Z.; Chen, X.; Liu, P.-F.; Wang, P.; Xu, Z.; Lin, Y.; Wang, B. Controlled Synthesis of Bifunctional NiCo2O4@FeNi LDH Core–Shell Nanoarray Air Electrodes for Rechargeable Zinc–Air Batteries. ACS Sustain. Chem. Eng. 2020, 8, 11079–11087. [Google Scholar] [CrossRef]
- Guo, X.; Hu, X.; Wu, D.; Jing, C.; Liu, W.; Ren, Z.; Zhao, Q.; Jiang, X.; Xu, C.; Zhang, Y.; et al. Tuning the Bifunctional Oxygen Electrocatalytic Properties of Core-Shell Co3O4@NiFe LDH Catalysts for Zn-Air Batteries: Effects of Interfacial Cation Valences. ACS Appl. Mater. Interfaces 2019, 11, 21506–21514. [Google Scholar] [CrossRef]
- Feng, X.; Jiao, Q.; Chen, W.; Dang, Y.; Dai, Z.; Suib, S.; Zhang, J.; Zhao, Y.; Li, H.; Feng, C. Cactus-like NiCo2S4@NiFe LDH hollow spheres as an effective oxygen bifunctional electrocatalyst in alkaline solution. Appl. Catal. B: Environ. 2021, 286, 119869. [Google Scholar] [CrossRef]
- Sumboja, A.; Chen, J.; Zong, Y.; Lee, P.; Liu, Z. NiMn layered double hydroxides as efficient electrocatalysts for the oxygen evolution reaction and their application in rechargeable Zn-air batteries. Nanoscale 2017, 9, 774–780. [Google Scholar] [CrossRef]
- Guo, X.; Zheng, T.; Ji, G.; Hu, N.; Xu, C.; Zhang, Y. Core/shell design of efficient electrocatalysts based on NiCo2O4 nanowires and NiMn LDH nanosheets for rechargeable zinc–air batteries. J. Mater. Chem. A 2018, 6, 10243–10252. [Google Scholar] [CrossRef]
- Li, K.; Guo, D.; Kang, J.; Wei, B.; Zhang, X.; Chen, Y. Hierarchical Hollow Spheres Assembled with Ultrathin CoMn Double Hydroxide Nanosheets as Trifunctional Electrocatalyst for Overall Water Splitting and Zn Air Battery. ACS Sustain. Chem. Eng. 2018, 6, 14641–14651. [Google Scholar] [CrossRef]
- Wang, T.; Wu, J.; Liu, Y.; Cui, X.; Ding, P.; Deng, J.; Zha, C.; Coy, E.; Li, Y. Scalable preparation and stabilization of atomic-thick CoNi layered double hydroxide nanosheets for bifunctional oxygen electrocatalysis and rechargeable zinc-air batteries. Energy Storage Mater. 2019, 16, 24–30. [Google Scholar] [CrossRef]
- Zhang, G.; Xing, J.; Zhao, Y.; Yang, F. Hierarchical N,P co-doped graphene aerogels framework assembling vertically grown CoMn-LDH nanosheets as efficient bifunctional electrocatalyst for rechargeable Zinc-air battery. J. Colloid. Interface Sci. 2021, 590, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Ataca, C.; Şahin, H.; Ciraci, S. Stable, Single-Layer MX2 Transition-Metal Oxides and Dichalcogenides in a Honeycomb-Like Structure. J. Phys. Chem. C 2012, 116, 8983–8999. [Google Scholar] [CrossRef]
- Geng, N.-N.D.; Hor, T.S.A.; Chien, S.W.; Liu, Z.; Zong, Y. Cobalt Sulfide Nanoparticles Impregnated Nitrogen and Sulfur co-Doped Graphene as Bifunctional Catalyst for Rechargeable Zn-air Batteries. RSC Adv. 2015, 5, 7280–7284. [Google Scholar] [CrossRef]
- Niu, W.; Li, Z.; Marcus, K.; Zhou, L.; Li, Y.; Ye, R.; Liang, K.; Yang, Y. Surface-Modified Porous Carbon Nitride Composites as Highly Efficient Electrocatalyst for Zn-Air Batteries. Adv. Energy Mater. 2018, 8, 1701642. [Google Scholar] [CrossRef]
- Jia, N.; Liu, J.; Gao, Y.; Chen, P.; Chen, X.; An, Z.; Li, X.; Chen, Y. Graphene-Encapsulated Co9S8 Nanoparticles on N,S-Codoped Carbon Nanotubes: An Efficient Bifunctional Oxygen Elec-trocatalyst. ChemSusChem 2019, 12, 3390–3400. [Google Scholar] [CrossRef]
- Sun, X.; Gong, Q.; Liang, Y.; Wu, M.; Xu, N.; Gong, P.; Sun, S.; Qiao, J. Exploiting a High-Performance "Double-Carbon" Structure Co9S8/GN Bifunctional Catalysts for Rechargeable Zn-Air Batteries. ACS Appl. Mater. Interfaces 2020, 12, 38202–38210. [Google Scholar] [CrossRef]
- Liu, T.; Yang, F.; Cheng, G.; Luo, W. Reduced Graphene Oxide-Wrapped Co9-xFexS8/Co,Fe-N-C Composite as Bifunctional Electrocatalyst for Oxygen Reduction and Evolution. Small 2018, 14, 1703748. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Dai, P.; Zhang, W.; Wu, M. Fish bone-derived N, S co-doped interconnected carbon nanofibers network coupled with (Fe, Co, Ni)9S8 nanoparticles as efficient bifunctional electrocatalysts for rechargeable and flexible all-solid-state Zn-air battery. Electrochimica Acta 2021, 373, 137903. [Google Scholar] [CrossRef]
- Chen, L.; Cui, L.; Wang, Z.; He, X.; Zhang, W.; Asefa, T. Co8FeS8/N,S-Doped Carbons Derived from Fe-Co/S-Bridged Polyphthalocyanine: Efficient Dual-Function Air-Electrode Catalysts for Rechargeable Zn-Air Batteries. ACS Sustain. Chem. Eng. 2020, 8, 13147–13158. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Balamurugan, J.; Vinothkannan, M.; Kim, A.; Sengodan, S.; Yoo, D. Nitrogen-doped graphene encapsulated FeCoMoS nanoparticles as advanced trifunctional catalyst for water splitting devices and zinc–air batteries. Appl. Catal. B: Environ. 2020, 279, 119381. [Google Scholar] [CrossRef]
- Zheng, X.; Han, X.; Liu, H.; Chen, J.; Fu, D.; Wang, J.; Zhong, C.; Deng, Y.; Hu, W. Controllable Synthesis of NixSe (0.5≤ x ≤ 1) Nanocrystals for Efficient Rechargeable Zinc-Air Batteries and Water Splitting. ACS Appl. Mater. Interfaces 2018, 10, 13675–13684. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Fung, V.; Sang, X.; Unocic, R.; Ganesh, P. Superior electrocatalytic hydrogen evolution at engineered non-stoichiometric two-dimensional transition metal dichalcogenide edges. J. Mater. Chem. A 2019, 7, 18357–18364. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, J.; Wang, J.; Zhang, Z.; Hu, W.; Han, Y. Facile synthesis of nickel cobalt selenide hollow nanospheres as efficient bifunctional electrocatalyst for rechargeable Zn-air battery. Sci. China Mater. 2019, 63, 347–355. [Google Scholar] [CrossRef]
- Cao, Y.; Zheng, X.; Zhang, H.; Zhang, J.; Han, X.; Zhong, C.; Hu, W.; Deng, Y. Interface engineering of NiS2/CoS2 nanohybrids as bifunctional electrocatalysts for rechargeable solid state Zn-air battery. J. Power Sources 2019, 437, 226893. [Google Scholar] [CrossRef]
- Yin, J.; Li, Y.; Lv, F.; Lu, M.; Sun, K.; Wang, W.; Wang, L.; Cheng, F.; Li, Y.; Xi, P.; et al. Oxygen Vacancies Dominated NiS2 /CoS2 Interface Porous Nanowires for Portable Zn-Air Batteries Driven Water Splitting Devices. Adv. Mater. 2017, 29, 1704681. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Deng, J.; Wang, G.; Hao, Y.; Bi, K.; Wang, X.; Yang, Y. P-doped CoS2 Embedded in TiO2 Nanoporous Films for Zn-Air Batteries. Adv. Funct. Mater. 2018, 28, 1804540. [Google Scholar] [CrossRef]
- Wang, X.; Sebastian, P.; Smit, M.; Yang, H.; Gamboa, S. Studies on the oxygen reduction catalyst for zinc–air battery electrode. J. Power Sources 2003, 124, 278–284. [Google Scholar] [CrossRef]
- Ma, B.H. A bifunctional electrocatalyst α-MnO2-LaNiO3/carbon nanotubes composite for rechargeable zinc-air batteries. RSC Adv. 2014, 4, 46084–46092. [Google Scholar] [CrossRef]
- Li, P.-C.; Hu, C.-C.; Lee, T.-C.; Chang, W.-S.; Wang, T. Synthesis and characterization of carbon black/manganese oxide air cathodes for zinc-air batteries. J. Power Sources 2014, 269, 88–97. [Google Scholar] [CrossRef]
- Sumboja, A.; Ge, X.; Goh, F.; Li, B.; Geng, D.; Hor, T.; Zong, Y.; Liu, Z. Manganese Oxide Catalyst Grown on Carbon Paper as an Air Cathode for High-Performance Rechargeable Zinc-Air Batteries. Chempluschem 2015, 80, 1341–1346. [Google Scholar] [CrossRef]
- Zhou, Q.; Hou, S.; Cheng, Y.; Sun, R.; Shen, W.; Tian, R.; Yang, J.; Pang, H.; Xu, L.; Huang, K.; et al. Interfacial engineering Co and MnO within N,S co-doped carbon hierarchical branched superstructures toward high-efficiency electrocatalytic oxygen reduction for robust Zn-air batteries. Appl. Catal. B Environ. 2021, 295, 120281. [Google Scholar] [CrossRef]
- Zhong, Y.; Dai, J.; Xu, X.; Su, C.; Shao, Z. Facilitating Oxygen Redox on Manganese Oxide Nanosheets by Tuning Active Species and Oxygen Defects for Zinc-Air Batteries. ChemElectroChem 2020, 7, 4949–4955. [Google Scholar] [CrossRef]
- Singh, S.; Dhavale, V.; Kurungot, S. Surface-Tuned Co3O4 Nanoparticles Dispersed on Nitrogen-Doped Graphene as an Efficient Cathode Electrocatalyst for Mechanical Rechargeable Zinc-Air Battery Application. ACS Appl. Mater. Interfaces 2015, 7, 21138–21149. [Google Scholar] [CrossRef] [PubMed]
- Guan, C.; Sumboja, A.; Wu, H.; Ren, W.; Liu, X.; Zhang, H.; Liu, Z.; Cheng, C.; Pennycook, S.; Wang, J. Hollow Co3O4 Nanosphere Embedded in Carbon Arrays for Stable and Flexible Solid-State Zinc-Air Batteries. Adv. Mater. 2017, 29, 1704117. [Google Scholar] [CrossRef]
- Ren, J.-T.; Yuan, G.-G.; Weng, C.-C.; Yuan, Z.-Y. Rationally Designed Co3O4–C Nanowire Arrays on Ni Foam Derived From Metal Organic Framework as Reversible Oxygen Evolution Electrodes with Enhanced Performance for Zn–Air Batteries. ACS Sustain. Chem. Eng. 2017, 6, 707–718. [Google Scholar] [CrossRef]
- Chong, Y.; Pan, Z.; Su, M.; Yang, X.; Ye, D.; Qiu, Y. 1D/2D hierarchical Co1-xFexO@N-doped carbon nanostructures for flexible zinc–air batteries. Electrochim. Acta 2020, 363, 137264. [Google Scholar] [CrossRef]
- Huang, K.; Wang, R.; Zhao, S.; Du, P.; Wang, H.; Wei, H.; Long, Y.; Deng, B.; Lei, M.; Ge, B.; et al. Atomic species derived CoOx clusters on nitrogen doped mesoporous carbon as advanced bifunctional electro-catalysts for Zn-air battery. Energy Storage Mater. 2020, 29, 156–162. [Google Scholar] [CrossRef]
- Wang, X.; Liao, Z.; Fu, Y.; Neumann, C.; Turchanin, A.; Nam, G.; Zschech, E.; Cho, J.; Zhang, J.; Feng, X. Confined growth of porous nitrogen-doped cobalt oxide nanoarrays as bifunctional oxygen electrocatalysts for rechargeable zinc–air batteries. Energy Storage Mater. 2020, 26, 157–164. [Google Scholar] [CrossRef]
- Wang, C.-C.; Hung, K.-Y.; Ko, T.-E.; Hosseini, S.; Li, Y.-Y. Carbon-nanotube-grafted and nano-Co3O4-doped porous carbon derived from metal-organic framework as an excellent bifunctional catalyst for zinc–air battery. J. Power Sources 2020, 452, 227841. [Google Scholar] [CrossRef]
- Prabu, M.; Ketpang, K.; Shanmugam, S. Hierarchical nanostructured NiCo2O4 as an efficient bifunctional non-precious metal catalyst for rechargeable zinc-air batteries. Nanoscale 2014, 6, 3173–3181. [Google Scholar]
- Prabu, M.; Ramakrishnan, P.; Shanmugam, S. CoMn2O4 nanoparticles anchored on nitrogen-doped graphene nanosheets as bifunctional electrocatalyst for rechargeable zinc—Air battery. Electrochem. Commun. 2014, 41, 59–63. [Google Scholar] [CrossRef]
- Lee, D.; Park, M.; Park, H.; Seo, M.; Ismayilov, V.; Ahmed, R.; Chen, Z. Highly active Co-doped LaMnO3 perovskite oxide and N-doped carbon nanotube hybrid bi-functional catalyst for rechargeable zinc—Air batteries. Electrochem. Commun. 2015, 60, 38–41. [Google Scholar] [CrossRef]
- Kosasang, S.; Gatemala, H.; Ma, N.; Chomkhuntod, P.; Sawangphruk, M. Trimetallic Spinel-Type Cobalt Nickel-Doped Manganese Oxides as Bifunctional Electrocatalysts for Zn-Air Batteries. Batter. Supercaps 2020, 3, 631–637. [Google Scholar] [CrossRef]
- Li, S.; Zhou, X.; Fang, G.; Xie, G.; Liu, X.; Lin, X.; Qiu, H.-J. Multicomponent Spinel Metal Oxide Nanocomposites as High-Performance Bifunctional Catalysts in Zn–Air Batteries. ACS Appl. Energy Mater. 2020, 3, 7710–7718. [Google Scholar] [CrossRef]
- Ran, J.; Wang, T.; Zhang, J.; Liu, Y.; Xu, C.; Xi, S.; Gao, D. Modulation of Electronics of Oxide Perovskites by Sulfur Doping for Electrocatalysis in Rechargeable Zn–Air Batteries. Chem. Mater. 2020, 32, 3439–3446. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, H.; Li, J.; Shi, Z.; Fan, M.; Bian, H.; Wang, T.; Gao, D. S-doped CoMn2O4 with more high valence metallic cations and oxygen defects for zinc-air batteries. J. Power Sources 2021, 491, 229584. [Google Scholar] [CrossRef]
- Chen, D.; Zhu, J.; Mu, X.; Cheng, R.; Li, W.; Liu, S.; Pu, Z.; Lin, C.; Mu, S. Nitrogen-Doped carbon coupled FeNi3 intermetallic compound as advanced bifunctional electrocatalyst for OER, ORR and zn-air batteries. Appl. Catal. B Environ. 2020, 268, 118729. [Google Scholar] [CrossRef]
- Yu, P.; Wang, L.; Sun, F.; Xie, Y.; Liu, X.; Ma, J.; Wang, X.; Tian, C.; Li, J.; Fu, H. Co Nanoislands Rooted on Co-N-C Nanosheets as Efficient Oxygen Electrocatalyst for Zn-Air Batteries. Adv. Mater. 2019, 31, e1901666. [Google Scholar] [CrossRef]
- He, X.; Tian, Y.; Huang, Z.; Xu, L.; Wu, J.; Qian, J.; Zhang, J.; Li, H. Engineering the electronic states of Ni3FeN via zinc ion regulation for promoting oxygen electrocatalysis in rechargeable Zn—Air batteries. J. Mater. Chem. A 2021, 9, 2301–2307. [Google Scholar] [CrossRef]
- Ji, H.; Wang, M.; Liu, S.; Sun, H.; Liu, J.; Qian, T.; Yan, C. Pyridinic and graphitic nitrogen-enriched carbon paper as a highly active bifunctional catalyst for Zn-air batteries. Electrochim. Acta 2020, 334, 135562. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, G.; Qiao, J.; Chen, N.; Chen, W.; Sun, S. Ultra-long life rechargeable zinc-air battery based on high-performance trimetallic nitride and NCNT hybrid bifunctional electrocatalysts. Nano Energy 2019, 61, 86–95. [Google Scholar] [CrossRef]
- Shinde, S.; Lee, C.; Sami, A.; Kim, D.; Lee, S.; Lee, J. Scalable 3-D Carbon Nitride Sponge as an Efficient Metal-Free Bifunctional Oxygen Electrocatalyst for Rechargeable Zn-Air Batteries. ACS Nano 2017, 11, 347–357. [Google Scholar] [CrossRef]
- Shinde, S.; Yu, J.; Song, J.; Nam, Y.; Kim, D.; Lee, J. Highly active and durable carbon nitride fibers as metal-free bifunctional oxygen electrodes for flexible Zn-air batteries. Nanoscale Horiz 2017, 2, 333–341. [Google Scholar] [CrossRef]
- Li, B.-Q.; Zhang, S.-Y.; Wang, B.; Xia, Z.-J.; Tang, C.; Zhang, Q. A porphyrin covalent organic framework cathode for flexible Zn–air batteries. Energy Environ. Sci. 2018, 11, 1723–1729. [Google Scholar] [CrossRef]
- Dinh, K.; Pei, Z.; Yuan, Z.; Hoang, V.; Wei, L.; Huang, Q.; Liao, X.; Liu, C.; Chen, Y.; Yan, Q. The on-demand engineering of metal-doped porous carbon nanofibers as efficient bifunctional oxygen catalysts for high-performance flexible Zn–air batteries. J. Mater. Chem. A 2020, 8, 7297–7308. [Google Scholar] [CrossRef]
- Qiao, Y.; Kong, F.; Zhang, C.; Li, R.; Kong, A.; Shan, Y. Highly efficient oxygen electrode catalyst derived from chitosan biomass by molten salt pyrolysis for zinc-air battery. Electrochim. Acta 2020, 339, 135923. [Google Scholar] [CrossRef]
- Wang, M.; Ji, H.; Liu, S.; Sun, H.; Liu, J.; Yan, C.; Qian, T. Single-atom scale metal vacancy engineering in heteroatom-doped carbon for rechargeable zinc-air battery with reduced overpotential. Chem. Eng. J. 2020, 393, 124702. [Google Scholar] [CrossRef]
- Luo, H.; Jiang, W.; Niu, S.; Zhang, X.; Zhang, Y.; Yuan, L.; He, C.; Hu, J. Self-Catalyzed Growth of Co-N-C Nanobrushes for Efficient Rechargeable Zn-Air Batteries. Small 2020, 16, e2001171. [Google Scholar] [CrossRef]
- Niu, Y.; Teng, X.; Gong, S.; Chen, Z. A bimetallic alloy anchored on biomass-derived porous N-doped carbon fibers as a self-supporting bifunctional oxygen electrocatalyst for flexible Zn—Air batteries. J. Mater. Chem. A 2020, 8, 13725–13734. [Google Scholar] [CrossRef]
- Yang, X.; Wu, X.; Guo, Z.; Li, Q.; Wang, H.; Ke, C.; Zeng, W.; Qiu, X.; He, Y.; Liang, X.; et al. Phosphorus/nitrogen co-doped and bimetallic MOF-derived cathode for all-solid-state rechargeable zinc—Air batteries. RSC Adv. 2020, 10, 33327–33333. [Google Scholar] [CrossRef]
- Amiinu, I.; Liu, X.; Pu, Z.; Li, W.; Li, Q.; Zhang, J.; Tang, H.; Zhang, H.; Mu, S. From 3D ZIF Nanocrystals to Co-Nx/C Nanorod Array Electrocatalysts for ORR, OER, and Zn-Air Batteries. Adv. Funct. Mater. 2018, 28, 1704638. [Google Scholar] [CrossRef]
- Liu, P.; Ran, J.; Xia, B.; Xi, S.; Gao, D.; Wang, J. Bifunctional Oxygen Electrocatalyst of Mesoporous Ni/NiO Nanosheets for Flexible Rechargeable Zn-Air Batteries. Nanomicro. Lett. 2020, 12, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Zhao, H.; Ma, Y.; Yang, Y.; Li, B.; Cui, Y.; Guo, Z.; Wang, L. Core-shell-structured Co@Co4N nanoparticles encapsulated into MnO-modified porous N-doping carbon nanocubes as bifunctional catalysts for rechargeable Zn–air batteries. J. Energy Chem. 2020, 50, 52–62. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, G.; Hu, Y.; Wang, J.; Sun, T.; Regier, T.; Qiao, J.; Sun, S. Graphitic-shell encapsulated FeNi alloy/nitride nanocrystals on biomass-derived N-doped carbon as an efficient electrocatalyst for rechargeable Zn-air battery. Carbon Energy 2020, 3, 176–187. [Google Scholar] [CrossRef]
- Yu, J.; Li, B.; Zhao, C.; Liu, J.; Zhang, Q. Asymmetric Air Cathode Design for Enhanced Interfacial Electrocatalytic Reactions in High-Performance Zinc-Air Batteries. Adv. Mater. 2020, 32, e1908488. [Google Scholar] [CrossRef]
- Fu, G.; Wang, J.; Chen, Y.; Liu, Y.; Tang, Y.; Goodenough, J.; Lee, J.-M. Exploring Indium-Based Ternary Thiospinel as Conceivable High-Potential Air-Cathode for Rechargeable Zn-Air Batteries. Adv. Energy Mater. 2018, 8, 1802263. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, J.; Bai, Z.; Jiang, G.; Li, M.; Feng, K.; Yang, L.; Ding, Y.; Yu, T.; Chen, Z.; et al. Controllable Urchin-Like NiCo2S4 Microsphere Synergized with Sulfur-Doped Graphene as Bifunctional Catalyst for Superior Rechargeable Zn-Air Battery. Adv. Funct. Mater. 2018, 28, 1706675. [Google Scholar] [CrossRef]
- Pan, Z.; Chen, H.; Yang, J.; Ma, Y.; Zhang, Q.; Kou, Z.; Ding, X.; Pang, Y.; Zhang, L.; Gu, Q.; et al. CuCo2S4 Nanosheets@N-Doped Carbon Nanofibers by Sulfurization at Room Temperature as Bifunctional Electrocatalysts in Flexible Quasi-Solid-State Zn-Air Batteries. Adv. Sci. 2019, 6, 1900628. [Google Scholar] [CrossRef]
- Balamurugan, J.; Nguyen, T.; Kim, D.; Kim, N.; Lee, J. 3D nickel molybdenum oxyselenide (Ni1-xMoxOSe) nanoarchitectures as advanced multifunctional catalyst for Zn-air batteries and water splitting. Appl. Catal. B: Environ. 2021, 286, 119909. [Google Scholar] [CrossRef]
- Shi, X.; He, B.; Zhao, L.; Gong, Y.; Wang, R.; Wang, H. FeS2–CoS2 incorporated into nitrogen-doped carbon nanofibers to boost oxygen electrocatalysis for durable rechargeable Zn-air batteries. J. Power Sources 2020, 482, 228955. [Google Scholar] [CrossRef]
- Wang, M.; Qian, T.; Zhou, J.; Yan, C. An Efficient Bifunctional Electrocatalyst for a Zinc-Air Battery Derived from Fe/N/C and Bimetallic Metal-Organic Framework Composites. ACS Appl. Mater. Interfaces 2017, 9, 5213–5221. [Google Scholar] [CrossRef]
- Wei, W.; Shi, X.; Gao, P.; Wang, S.; Hu, W.; Zhao, X.; Ni, Y.; Xu, X.; Xu, Y.; Yan, W.; et al. Well-elaborated, mechanochemically synthesized Fe-TPP⊂ZIF precursors (Fe-TPP = tetraphenylporphine iron) to atomically dispersed iron–nitrogen species for oxygen reduction reaction and Zn-air batteries. Nano Energy 2018, 52, 29–37. [Google Scholar] [CrossRef]
- He, Y.; Liu, X.; Yan, A.; Wan, H.; Chen, G.; Pan, J.; Zhang, N.; Qiu, T.; Ma, R.; Qiu, G. Hybrid Nanostructures of Bimetallic NiCo Nitride/N-Doped Reduced Graphene Oxide as Efficient Bifunctional Electrocatalysts for Rechargeable Zn–Air Batteries. ACS Sustain. Chem. Eng. 2019, 7, 19612–19620. [Google Scholar] [CrossRef]
- Lin, C.; Li, X.; Shinde, S.; Kim, D.-H.; Song, X.; Zhang, H.; Lee, J.-H. Long-Life Rechargeable Zn Air Battery Based on Binary Metal Carbide Armored by Nitrogen-Doped Carbon. ACS Appl. Energy Mater. 2019, 2, 1747–1755. [Google Scholar] [CrossRef]
- Wagh, N.; Shinde, S.; Lee, C.; Jung, J.-Y.; Kim, D.-H.; Kim, S.-H.; Lin, C.; Lee, S.; Lee, J.-H. Densely colonized isolated Cu-N single sites for efficient bifunctional electrocatalysts and rechargeable advanced Zn-air batteries. Appl. Catal. B: Environ. 2020, 268, 118746. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, X.; Yang, M.; Shi, Y.; Liu, W.; Lin, S. Constructing Co@WC1-x heterostructure on N-doped carbon nanotubes as an efficient bifunctional electrocatalyst for zinc-air batteries. J. Power Sources 2020, 485, 229251. [Google Scholar] [CrossRef]
- Li, Y.; Xu, K.; Zhang, Q.; Zheng, Z.; Li, S.; Zhao, Q.; Li, C.; Dong, C.; Mei, Z.; Pan, F.; et al. One-pot synthesis of FeNxC as efficient catalyst for high-performance zinc-air battery. J. Energy Chem. 2022, 66, 100–106. [Google Scholar] [CrossRef]
- Xu, W.; Lu, Z.; Zhang, T.; Zhong, Y.; Wu, Y.; Zhang, G.; Liu, J.; Wang, H.; Sun, X. An advanced zinc air battery with nanostructured superwetting electrodes. Energy Storage Mater. 2019, 17, 358–365. [Google Scholar] [CrossRef]
- Zhao, J.; He, Y.; Chen, Z.; Zheng, X.; Han, X.; Rao, D.; Zhong, C.; Hu, W.; Deng, Y. Engineering the Surface Metal Active Sites of Nickel Cobalt Oxide Nanoplates toward Enhanced Oxygen Electrocatalysis for Zn-Air Battery. ACS Appl. Mater. Interfaces 2019, 11, 4915–4921. [Google Scholar] [CrossRef]
- Majee, R.; Das, T.; Chakraborty, S.; Bhattacharyya, S. Shaping a Doped Perovskite Oxide with Measured Grain Boundary Defects to Catalyze Bifunctional Oxygen Activation for a Rechargeable Zn-Air Battery. ACS Appl. Mater. Interfaces 2020, 12, 40355–40363. [Google Scholar] [CrossRef]
- Hong, J.; Kim, J.; Park, G.; Lee, J.; Lee, J.-K.; Kang, Y. A strategy for fabricating three-dimensional porous architecture comprising metal oxides/CNT as highly active and durable bifunctional oxygen electrocatalysts and their application in rechargeable Zn-air batteries. Chem. Eng. J. 2021, 414, 128815. [Google Scholar] [CrossRef]



| Installation Cost ($/kW) | LCOE $/MWh | LCOE $/kW yr | ||
|---|---|---|---|---|
| Zn-Air | 50 MW/6 h | 3200 | 160 | 355 |
| 1 MW/6 h | 3900 | 200 | 440 | |
| Lead-Acid | 50 MW/5 h | 3800 | 220 | 410 |
| Na/S | 50 MW/6 h | 5780 | 295 | 645 |
| 1 MW/7.2 h | 6100 | 280 | ~735 |
| Number | Catalyst | Overpotential/V (10 mA cm−2) | Tafel Slope/mV dec−1 | Electrolyte | Catalyst Loadings (mg cm−2) | Ref |
|---|---|---|---|---|---|---|
| Noble metals | ||||||
| 1 | Ni3FeN | 0.38 | 0.1 M KOH | 0.12 | [15] | |
| 2 | Fe3Pt/Ni3FeN | 0.365 | 0.1 M KOH | 0.12 | [15] | |
| 3 | Co(OH)2/CoPt/N-CN | 0.32 | 73.4 | 1 M KOH | 0.102 | [17] |
| 4 | SA-PtCoF | 0.308 | 68 | 1 M KOH | 0.42 | [18] |
| 5 | Pt/CoFe2O4 | 0.28 | 88 | 1 M KOH | 0.283 | [19] |
| 7 | Ag/LMO-NR/RGO | 0.49 | 0.1 M KOH | 0.317 | [22] | |
| 8 | Ag-Cu/Ni | 0.27 | 0.1 M KOH | 0.8 | [23] | |
| 9 | Ru0.7Sn0.3O2 | 0.24 | 0.1 M KOH | 0.1 | [25] | |
| N-doped carbonaceous (NC) materials | ||||||
| 10 | NDGs-800 | 0.45 | 132 | 1 M KOH | 0.4 | [34] |
| 11 | SilkNC/KB | 0.73 | 109 | 0.1 M KOH | 0.6 | [35] |
| 12 | GNCNTs-4 | 0.37 | 61 | 0.1 M KOH | 0.51 | [37] |
| 13 | NPC@CNF-950 | 0.47 | 132 | 0.1 M KOH | 0.1 | [36] |
| 14 | Honeycomb-600 | 0.38 | 85.9 | 0.1 M KOH | 0.3 | [38] |
| 15 | 3D Fe/N-G | 0.393 | 71.5 | 0.1 M KOH | 0.276 | [43] |
| 16 | Fe-MNC | 0.348 | 127 | 1 M KOH | 0.286 | [44] |
| 17 | 2D Fe-NG | 0.39 | 70.1 | 0.1 M KOH | 0.236 | [45] |
| 18 | hSNCNC | 0.41 | 288 | 0.1 M KOH | 0.127 | [52] |
| Metallic nanoparticle | ||||||
| 19 | Co-N-CNTs | 0.46 | 0.1 M KOH | 0.81 | [60] | |
| 20 | CNCF-800 | 0.41 | 0.1 M KOH | 0.25 | [61] | |
| 21 | Co@NCNTA-700 | 0.28 | 0.1 M KOH | 1 | [62] | |
| 22 | Co@NC-3 | 0.49 | 92 | 0.1 M KOH | 0.24 | [63] |
| 23 | Co/N-CNTs@rGO | 0.381 | 0.1 M KOH | [64] | ||
| 24 | Co/Co–N–C | 0.47 | 0.1 M KOH | 0.382 | [140] | |
| 25 | Co@NC-5 | 0.390 | 0.1 M KOH | 0.5 | [65] | |
| 26 | FeCo@NC-750 | 0.22 | 52 | 0.1 M KOH | 0.4 | [75] |
| 27 | h-FeCo alloy/N-CNFs | 0.368 | 62.74 | 1 M KOH | 0.2 | [76] |
| 28 | CoFe@NCNTs | 0.45 | 158 | 0.1 M KOH | 0.8 | [77] |
| 29 | Fe1.2Co@NC/NCNTs | 0.355 | 66 | 0.1 M KOH | 0.2 | [78] |
| 30 | Ni3Fe/N-C | 0.310 | 58 | 1 M KOH | 0.22 | [80] |
| 31 | Ni3Fe/NPG-1 | 0.329 | 82 | 0.1 M KOH | 1 | [81] |
| 32 | Fe-enriched-FeNi3/NC | 0.36 | 82 | 0.1 M KOH | 0.8 | [82] |
| 33 | Ni3Fe/N-S-CNTs | 0.215 | 44.1 | 1 M KOH | 0.42 | [85] |
| 34 | FeNi/N-CPCF-950 | 0.355 | 67 | 0.1 M KOH | 0.6 | [84] |
| 35 | NiFe@NCNTs (Ni0.36Fe0.64) | 0.33 | 74 | 1 M KOH | 0.408 | [83] |
| 36 | FeNi3@NC | 0.277 | 77 | 1 M KOH | -- | [139] |
| Layered double hydroxide | ||||||
| 37 | MCN-LDH-1.5 | 0.44 | 140 | 0.1 M KOH | 0.4 | [94] |
| 38 | Co3O4@NiFe-2 | 0.31 | 55.5 | 1 M KOH | 0.4 | [97] |
| 39 | NiCo2S4@NiFe | 0.287 | 86.4 | 0.1 M KOH | -- | [98] |
| 40 | NiMn (Ni3Mn1) | 0.38 | 40 | 1 M KOH | 0.2 | [99] |
| 41 | NiCo2O4@NiMn | 0.255 | 62.4 | 1 M KOH | 2.0 ± 0.2 | [100] |
| 42 | Hollow sphere Co2Mn1 | 0.255 | 57 | 1 M KOH | 2.0 | [101] |
| 43 | CoNi-NS/rGO | 0.33 | 62 | 1 M KOH | 0.48 | [102] |
| 44 | CoMn-LDH/NPGA | 0.31 | 64.1 | 0.1 M KOH | 0.255 | |
| Metal chalcogenides | ||||||
| 45 | CoSx@PCN/rGO | 0.34 | 44 | 0.1 M KOH | 0.408 | [106] |
| 46 | Co/N/S-800 | 0.361 | 102 | 0.1 M KOH | 0.1 | [107] |
| 47 | S-Co9−xFexS8@rGO | 0.29 | 66 | 0.1 M KOH | 0.5 | [109] |
| 48 | FeCoMoS@NG | 0.238 | 51 | 0.1 M KOH | 0.21 | [112] |
| 49 | (Fe,Co)SPPc-900-sp | 0.353 | 53 | 1 M KOH | 0.28 | [111] |
| 50 | Ni0.5Se | 0.33 | 51 | 1 M KOH | [113] | |
| 51 | NiS2/CoS2 | 0.295 | 51 | 0.1 M KOH | [116] | |
| 52 | NiS2/CoS2–O NWs | 0.235 | 31 | 1 M KOH | [117] | |
| 53 | N, P/CoS2@TiO2 | 0.26 | 79 | 0.1 M KOH | [118] | |
| Metal oxides | ||||||
| 54 | NC-Co3O4-90 | 0.358 | 1 M KOH | [126] | ||
| 55 | CoOx/NMC | 0.269 | 59.8 | 1 M KOH | [129] | |
| 56 | NP-Co3O4/CC | 0.330 | 1 M KOH | [130] | ||
| 57 | S5.84%-LaCoO3 | 0.364 | 126.7 | 1 M KOH | [137] | |
| 58 | S–CoMn2O4 | 0.35 | 88 | 1 M KOH | [138] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phuc, N.H.H.; Anh Tu, T.; Cam Loc, L.; Xuan Viet, C.; Thi Thuy Phuong, P.; Tri, N.; Van Thang, L. A Review of Bifunctional Catalysts for Zinc-Air Batteries. Nanoenergy Adv. 2023, 3, 13-47. https://doi.org/10.3390/nanoenergyadv3010003
Phuc NHH, Anh Tu T, Cam Loc L, Xuan Viet C, Thi Thuy Phuong P, Tri N, Van Thang L. A Review of Bifunctional Catalysts for Zinc-Air Batteries. Nanoenergy Advances. 2023; 3(1):13-47. https://doi.org/10.3390/nanoenergyadv3010003
Chicago/Turabian StylePhuc, Nguyen Huu Huy, Tran Anh Tu, Luu Cam Loc, Cao Xuan Viet, Pham Thi Thuy Phuong, Nguyen Tri, and Le Van Thang. 2023. "A Review of Bifunctional Catalysts for Zinc-Air Batteries" Nanoenergy Advances 3, no. 1: 13-47. https://doi.org/10.3390/nanoenergyadv3010003