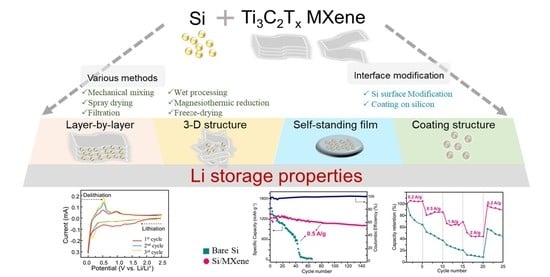
Enhanced Performance of Silicon Negative Electrodes Composited with Titanium Carbide Based MXenes for Lithium-Ion Batteries
Abstract
:1. Introduction
1.1. Silicon Based Negative Electrode Materials for LIBs
1.2. Silicon/2D Material Composites
1.3. Application of MXenes in LIBs
2. Structures and Preparation Strategies of Si/MXene Composites as Negatrodes in LIBs
2.1. Structures of Si/MXene Composites
2.1.1. Layer-by-Layer Structure
2.1.2. Self-Standing Film
2.1.3. Three-Dimensional Structure
2.1.4. Coating Structure
2.2. Preparation Methods
2.2.1. Mechanical Mixing
2.2.2. Wet Processing Method
2.2.3. Spray Drying Method
2.2.4. Magnesiothermic Reduction Method
2.2.5. Filtration Method
2.2.6. Freeze-Drying Method
2.3. Interface Modification of Si in Si/MXene Composites
2.3.1. Si Surface Modification—Positive Charged
2.3.2. Coating on the Surface of the Silicon
3. Electrochemical Performance of Si/MXene Composites as Negatrodes in LIBs
| Materials | Preparation Methods | Structure | Cycle Stability | Rate Capability | ICE | Ref. |
|---|---|---|---|---|---|---|
| Si@Ti3C2 MXene | Mechanical mixing | Layer-by-Layer | 0.2 A g−1, 150 cycles, 188 mAh g−1 | 3 A g−1, ~100 mAh g−1 | 69% | [47] |
| MXene bonded Si@C film | Filtration | Free-standing film | 420 mA g−1, 150 cycles, 1041 mAh g−1 | 8.4 A g−1, ~500 mAh g−1 | 73% | [53] |
| MXene-Si-CNT | Mechanical mixing | 3D Porous | 2 A g−1, 200 cycles, 841 mAh g−1 | 2 A g−1, ~800 mAh g−1 | 70.38% | [60] |
| MXene&Si | Filtration | Free-standing film | 100 mA g−1, 500 cycles, 558 mAh g−1 | 5 C, ~150 mAh g−1 | 61% | [52] |
| Si/d-Ti3C2 | Freeze-drying | Layer-by-Layer | 500 mA g−1, 200 cycles, 1130 mAh g−1 | 2 A g−1, 890 mAh g−1 | 74.10% | [46] |
| Si/MXene | Filtration | Free-standing film | 1 A g−1, 200 cycles, 1672 mAh·g−1 | 5 A g−1, 886 mAh g−1 | 71% | [54] |
| nSi/MX-C | Mechanical mixing | Layer-by-Layer | 1.5 A g−1, 70 cycles, 1106 mAh g−1 | 3 A g−1, 1300 mAh g−1 | 81–83% | [49] |
| Ti3C2/Si | Magnesiothermic reduction | 3D porous | 1 A g−1, 800 cycles, 956 mAh g−1 | 2 A g−1, ~450 mAh g−1 | 61.10% | [56] |
| SiO2/MXene | Spray drying | Coating | 1 A g−1, 200 cycles, 635 mAh g−1 | 3 A g−1, ~500 mAh g−1 | 71% | [68] |
| MXene/Si@SiOx@C | Magnesiothermic reduction | Layer-by-Layer | 10 C, 1000 cycles, 390 mAh g−1 | 10 C, ~400 mAh g−1 | 81.30% | [48] |
| NH2-Si/Ti3C2Tx | Wet processing | Layer-by-Layer | 0.1 C, 100 cycles, 864 mAh g−1 | 5 C, ~100 mAh g−1 | 75.20% | [41] |
| Si@Ti3C2 | Wet processing | Layer-by-Layer | 1 A g−1, 200 cycles, 1343 mAh g−1 | 3 A g−1, ~1500 mAh g−1 | 73.40% | [50] |
| Ti3C2@Si/SiOx@TiO2 | Magnesiothermic reduction | Sandwiched | 0.1 A g−1, 100 cycles, 939 mAh g−1 | 66.30% | [61] | |
| Ti3C2Tx/10%Si scrolls | Freeze-drying | Scroll | 400 mA g−1, 600 cycles, ~200 mAh g−1 | 5 A g−1, ~80 mAh g−1 | [63] | |
| NH2-Si/Ti3C2Tx | Wet processing | Layer-by-Layer | 0.3 A g−1, 100 cycles, 644 mAh g−1 | 72.70% | [45] | |
| SiO/wrinkled MXene | Wet processing | 3D Porous | 0.3 A g−1, 100 cycles, ~850 mAh g−1 | 2 A g−1, ~1000 mAh g−1 | 69.40% | [76] |
| Ti3C2Tx-CNT/SiNPs | Filtration | Free-standing film | 0.1 A g−1, 150 cycles, 2.18 mAh cm−2 | 2 A g−1, ~1 mAh cm−2 | 62.80% | [55] |
| SiNP@MX1/MX2 | Freeze-drying | 3D Porous | 0.5 A g−1, 200 cycles, 1422 mAh g−1 | 5 A g−1, ~500 mAh g−1 | 67.20% | [59] |
| Si p-NS@TNSs | Mechanical mixing | Coating | 0.2 A g−1, 150 cycles, 1154 mAh g−1 | 80.20% | [64] | |
| MXene/L-Si/C | Filtration | Layer-by-Layer | 500 mA g−1, 500 cycles, ~100 mAh g−1 | ~82% | [51] | |
| Si@NC/MX | Wet processing | 3D Porous | 1 A g−1, 300 cycles, 953 mAh g−1 | 10 A g−1, ~900 mAh g−1 | 75% | [57] |
| Si/MXene@CNFs | Electrospinning | Fiber | 1 A g−1, 200 cycles, 440 mAh g−1 | 5 A g−1, ~500 mAh g−1 | [62] | |
| Si@MXene | Freeze-drying | Coating | 0.2 A g−1, 100 cycles, 981 mAh g−1 | 2 A g−1, ~1000 mAh g−1 | 71.30% | [66] |
| Si@TiO2-TiSi2 | Spray drying | Coating (Core-shell) | 2 A g−1, 100 cycles, 1004 mAh g−1 | ~91% | [67] | |
| Si@Ti3C2Tx | Freeze-drying | 3D Porous | 1 A g−1, 500 cycles, 1729 mAh g−1 | 2 A g−1, ~500 mAh g−1 | [58] | |
| Si@MXene | Spray drying | Coating | 2 A g−1, 500 cycles, 400 mAh g−1 | [65] | ||
| Si-N-MXene | Mechanical mixing | 3D Porous | 3.2 A g−1, 900 cycles, 400 mAh g−1 | 6.4 A g−1, 1469 mAh g−1 | ~85% | [37] |
4. Conclusions and Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Wang, F.; Wu, X.; Li, C.; Zhu, Y.; Fu, L.; Wu, Y.; Liu, X. Nanostructured positive electrode materials for post-lithium ion batteries. Energy Environ. Sci. 2016, 9, 3570–3611. [Google Scholar] [CrossRef]
- Han, G.-B.; Ryou, M.-H.; Cho, K.Y.; Lee, Y.M.; Park, J.-K. Effect of succinic anhydride as an electrolyte additive on electrochemical characteristics of silicon thin-film electrode. J. Power Sources 2010, 195, 3709–3714. [Google Scholar] [CrossRef]
- Wang, T.; Ji, X.; Wu, F.; Yang, W.; Dai, X.; Xu, X.; Wang, J.; Guo, D.; Chen, M. Facile fabrication of a three-dimensional coral-like silicon nanostructure coated with a C/rGO double layer by using the magnesiothermic reduction of silica nanotubes for high-performance lithium-ion battery anodes. J. Alloys Compd. 2021, 863, 158569. [Google Scholar] [CrossRef]
- Huang, S.; Qin, X.; Lei, C.; Miao, X.; Wei, T. A one-pot method to fabricate reduced graphene oxide (rGO)-coated Si@SiOx@β-Bi2O3/Bi composites for lithium-ion batteries. Electrochim. Acta 2021, 390, 138857. [Google Scholar] [CrossRef]
- Lu, Q.; Jeong, B.-G.; Peng, X.; Jeong, S.-W.; Xie, B.; Wu, Z. The robust carbon shell to improve stability of porous Si anodes for high-performance lithium-ion batteries. J. Alloys Compd. 2021, 877, 160193. [Google Scholar] [CrossRef]
- Liu, X.H.; Liu, Y.; Kushima, A.; Zhang, S.; Zhu, T.; Li, J.; Huang, J.Y. In Situ TEM Experiments of Electrochemical Lithiation and Delithiation of Individual Nanostructures. Adv. Energy Mater. 2012, 2, 722–741. [Google Scholar] [CrossRef]
- McDowell, M.T.; Lee, S.W.; Nix, W.D.; Cui, Y. 25th anniversary article: Understanding the lithiation of silicon and other alloying anodes for lithium-ion batteries. Adv. Mater. 2013, 25, 4966–4985. [Google Scholar] [CrossRef]
- Obrovac, M.N.; Krause, L.J. Reversible Cycling of Crystalline Silicon Powder. J. Electrochem. Soc. 2007, 154, A103–A108. [Google Scholar] [CrossRef]
- Rhodes, K.; Dudney, N.; Lara-Curzio, E.; Daniel, C. Understanding the Degradation of Silicon Electrodes for Lithium-Ion Batteries Using Acoustic Emission. J. Electrochem. Soc. 2010, 157, A1354–A1360. [Google Scholar] [CrossRef]
- Ryu, J.; Chen, T.; Bok, T.; Song, G.; Ma, J.; Hwang, C.; Luo, L.; Song, H.K.; Cho, J.; Wang, C.; et al. Mechanical mismatch-driven rippling in carbon-coated silicon sheets for stress-resilient battery anodes. Nat. Commun. 2018, 9, 2924. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.-G.; Hu, J.-S.; Wan, L.-J. Nanostructured Materials for Electrochemical Energy Conversion and Storage Devices. Adv. Mater. 2008, 20, 2878–2887. [Google Scholar] [CrossRef]
- Kwon, H.J.; Hwang, J.Y.; Shin, H.J.; Jeong, M.G.; Chung, K.Y.; Sun, Y.K.; Jung, H.G. Nano/Microstructured Silicon-Carbon Hybrid Composite Particles Fabricated with Corn Starch Biowaste as Anode Materials for Li-Ion Batteries. Nano Lett. 2020, 20, 625–635. [Google Scholar] [CrossRef]
- Jin, H.; Zhu, M.; Liu, J.; Gan, L.; Gong, Z.; Long, M. Alkaline chitosan solution as etching phase to design Si@SiO2@N-Carbon anode for Lithium-ion battery. Appl. Surf. Sci. 2021, 541, 148436. [Google Scholar] [CrossRef]
- Zhou, X.; Xie, H.; He, X.; Zhao, Z.; Ma, Q.; Cai, M.; Yin, H. Annihilating the Formation of Silicon Carbide: Molten Salt Electrolysis of Carbon–Silica Composite to Prepare the Carbon–Silicon Hybrid for Lithium-Ion Battery Anode. Energy Environ. Mater. 2020, 3, 166–176. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Hou, Z.; Zhang, X.; Zhang, L.; Li, C. Delicate construction of Si@SiOx composite materials by microwave hydrothermal for lithium-ion battery anodes. Ionics 2019, 26, 69–74. [Google Scholar] [CrossRef]
- Guo, J.; Zhai, W.; Sun, Q.; Ai, Q.; Li, J.; Cheng, J.; Dai, L.; Ci, L. Facilely tunable core-shell Si@SiOx nanostructures prepared in aqueous solution for lithium ion battery anode. Electrochim. Acta 2020, 342, 136068. [Google Scholar] [CrossRef]
- Chen, Q.; Tan, L.; Wang, S.; Liu, B.; Peng, Q.; Luo, H.; Jiang, P.; Tang, H.; Sun, R. A facile synthesis of phosphorus doped Si/SiO2/C with high coulombic efficiency and good stability as an anode material for lithium ion batteries. Electrochim. Acta 2021, 385, 138385. [Google Scholar] [CrossRef]
- Wu, J.; Liu, J.; Wang, Z.; Gong, X.; Wang, Y. A new design for Si wears double jackets used as a high-performance lithium-ion battery anode. Chem. Eng. J. 2019, 370, 565–572. [Google Scholar] [CrossRef]
- Xiang, J.; Liu, H.; Na, R.; Wang, D.; Shan, Z.; Tian, J. Facile preparation of void-buffered Si@TiO2/C microspheres for high-capacity lithium ion battery anodes. Electrochim. Acta 2020, 337, 135841. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, H.-R.; Seo, H.; Kim, K.; Kim, J.-H. Novel synthesis of porous Si-TiO2 composite as a high-capacity anode material for Li secondary batteries. J. Alloys Compd. 2021, 872, 159640. [Google Scholar] [CrossRef]
- Cai, X.; Lai, L.; Shen, Z.; Lin, J. Graphene and graphene-based composites as Li-ion battery electrode materials and their application in full cells. J. Mater. Chem. A 2017, 5, 15423–15446. [Google Scholar] [CrossRef]
- Wang, D.; Guo, G.-C.; Wei, X.-L.; Liu, L.-M.; Zhao, S.-J. Phosphorene ribbons as anode materials with superhigh rate and large capacity for Li-ion batteries. J. Power Sources 2016, 302, 215–222. [Google Scholar] [CrossRef]
- Naresh, K.J.; Rafael, B.A.; Vivekanand, S.; Rajeev, A. Borophane as a Bench-mate of Graphene: A Potential 2D Material for Anode of Li and Na-ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 16148–16158. [Google Scholar]
- Zhao, G.; Cheng, Y.; Sun, P.; Ma, W.; Hao, S.; Wang, X.; Xu, X.; Xu, Q.; Liu, M. Biocarbon based template synthesis of uniform lamellar MoS2 nanoflowers with excellent energy storage performance in lithium-ion battery and supercapacitors. Electrochim. Acta 2020, 331, 135262. [Google Scholar] [CrossRef]
- Folorunso, O.; Kumar, N.; Hamam, Y.; Sadiku, R.; Ray, S.S. Recent progress on 2D metal carbide/nitride (MXene) nanocomposites for lithium-based batteries. FlatChem 2021, 29, 100281. [Google Scholar] [CrossRef]
- Du, L.; Lin, H.; Ma, Z.; Wang, Q.; Li, D.; Shen, Y.; Zhang, W.; Rui, K.; Zhu, J.; Huang, W. Using and recycling V2O5 as high performance anode materials for sustainable lithium ion battery. J. Power Sources 2019, 424, 158–164. [Google Scholar] [CrossRef]
- Yang, J.; Liu, J.; Zhao, C.; Zhang, W.; Li, X. Core-shell structured heterohierachical porous Si@graphene microsphere for high-performance lithium-ion battery anodes. Mater. Lett. 2020, 266, 127484. [Google Scholar] [CrossRef]
- Han, X.; Zhang, Z.; Chen, H.; Zhang, Q.; Chen, S.; Yang, Y. On the Interface Design of Si and Multilayer Graphene for a High-Performance Li-Ion Battery Anode. ACS Appl. Mater. Interfaces 2020, 12, 44840–44849. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, H.-X.; Wu, Y.-Q.; Pu, H.; Meng, W.-J.; Gao, R.-Z.; Zhao, D.-L. Graphene caging core-shell Si@Cu nanoparticles anchored on graphene sheets for lithium-ion battery anode with enhanced reversible capacity and cyclic performance. Electrochim. Acta 2020, 341, 136037. [Google Scholar] [CrossRef]
- Srimuk, P.; Kaasik, F.; Krüner, B.; Tolosa, A.; Fleischmann, S.; Jäckel, N.; Tekeli, M.C.; Aslan, M.; Suss, M.E.; Presser, V. MXene as a novel intercalation-type pseudocapacitive cathode and anode for capacitive deionization. J. Mater. Chem. A 2016, 4, 18265–18271. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Chen, X.; Xiang, P.; Du, H.; Xiao, B. Chalcogenated-Ti3C2X2 MXene (X = O, S, Se and Te) as a high-performance anode material for Li-ion batteries. Appl. Surf. Sci. 2020, 501, 144221. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, Z.; Huang, H.; Chu, X.; Xie, Y.; Xiong, D.; Yan, C.; Zhao, H.; Zhang, H.; Yang, W. Unraveling and Regulating Self-Discharge Behavior of Ti3C2Tx MXene-Based Supercapacitors. ACS Nano 2020, 14, 4916–4924. [Google Scholar] [CrossRef] [PubMed]
- Yun, T.; Kim, H.; Iqbal, A.; Cho, Y.S.; Lee, G.S.; Kim, M.K.; Kim, S.J.; Kim, D.; Gogotsi, Y.; Kim, S.O.; et al. Electromagnetic Shielding of Monolayer MXene Assemblies. Adv. Mater. 2020, 32, 1906769. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lee, J.-M. Recent advances in structural engineering of MXene electrocatalysts. J. Mater. Chem. A 2020, 8, 10604–10624. [Google Scholar] [CrossRef]
- Lei, J.-C.; Zhang, X.; Zhou, Z. Recent advances in MXene: Preparation, properties, and applications. Front. Phys. 2015, 10, 276–286. [Google Scholar] [CrossRef]
- Huang, S.; Mutyala, K.C.; Sumant, A.V.; Mochalin, V.N. Achieving superlubricity with 2D transition metal carbides (MXenes) and MXene/graphene coatings. Mater. Today Adv. 2021, 9, 100133. [Google Scholar] [CrossRef]
- Han, X.; Zhou, W.; Chen, M.; Chen, J.; Wang, G.; Liu, B.; Luo, L.; Chen, S.; Zhang, Q.; Shi, S.; et al. Interfacial nitrogen engineering of robust silicon/MXene anode toward high energy solid-state lithium-ion batteries. J. Energy Chem. 2022, 67, 727–735. [Google Scholar] [CrossRef]
- Gou, L.; Jing, W.; Li, Y.; Wang, M.; Hu, S.; Wang, H.; He, Y.-B. Lattice-Coupled Si/MXene Confined by Hard Carbon for Fast Sodium-Ion Conduction. ACS Appl. Energy Mater. 2021, 4, 7268–7277. [Google Scholar] [CrossRef]
- Ahmed, B.; Anjum, D.H.; Gogotsi, Y.; Alshareef, H.N. Atomic layer deposition of SnO2 on MXene for Li-ion battery anodes. Nano Energy 2017, 34, 249–256. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Xie, X.; Anasori, B.; Sarycheva, A.; Makaryan, T.; Zhao, M.; Urbankowski, P.; Miao, L.; Jiang, J.; Gogotsi, Y. MoS2 -on-MXene Heterostructures as Highly Reversible Anode Materials for Lithium-Ion Batteries. Angew. Chem. Int. Ed. Engl. 2018, 57, 1846–1850. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, J.; Wang, X.; Qin, J.; Cao, M. A Hybrid Assembly of MXene with NH2 -Si Nanoparticles Boosting Lithium Storage Performance. Chem. Asian J. 2020, 15, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Matios, E.; Wang, H.; Tao, X.; Li, W. Interfacial structure design of MXene-based nanomaterials for electrochemical energy storage and conversion. InfoMat 2020, 2, 1057–1076. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, H.L.; Li, Z.; Shan, H.; Scott, X.M.; Ting, Z.; Jian, Y.H. Size-Dependent Fracture of Silicon Nanoparticles During Lithiation. ACS Nano 2012, 6, 1522–1531. [Google Scholar]
- Zhang, F.; Jia, Z.; Wang, C.; Feng, A.; Wang, K.; Hou, T.; Liu, J.; Zhang, Y.; Wu, G. Sandwich-like silicon/Ti3C2Tx MXene composite by electrostatic self-assembly for high performance lithium ion battery. Energy 2020, 195, 117047. [Google Scholar] [CrossRef]
- Zhu, X.; Shen, J.; Chen, X.; Li, Y.; Peng, W.; Zhang, G.; Zhang, F.; Fan, X. Enhanced cycling performance of Si-MXene nanohybrids as anode for high performance lithium ion batteries. Chem. Eng. J. 2019, 378, 122212. [Google Scholar] [CrossRef]
- Kong, F.; He, X.; Liu, Q.; Qi, X.; Sun, D.; Zheng, Y.; Wang, R.; Bai, Y. Enhanced reversible Li-ion storage in Si@Ti3C2 MXene nanocomposite. Electrochem. Commun. 2018, 97, 16–21. [Google Scholar] [CrossRef]
- Zhang, Y.; Mu, Z.; Lai, J.; Chao, Y.; Yang, Y.; Zhou, P.; Li, Y.; Yang, W.; Xia, Z.; Guo, S. MXene/Si@SiOx@C Layer-by-Layer Superstructure with Autoadjustable Function for Superior Stable Lithium Storage. ACS Nano 2019, 13, 2167–2175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.J.; Park, S.-H.; Seral-Ascaso, A.; Barwich, S.; McEvoy, N.; Boland, C.S.; Coleman, J.N.; Gogotsi, Y.; Nicolosi, V. High capacity silicon anodes enabled by MXene viscous aqueous ink. Nat. Commun. 2019, 10, 849. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Wang, Z.; Xia, Y.; Wu, G.; Chen, C.; Wang, J.; Rao, P.; Dong, A. Facile electrostatic assembly of Si@MXene superstructures for enhanced lithium-ion storage. J. Colloid Interface Sci. 2020, 580, 68–76. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Tian, Y.; Zhang, Y.; Wei, C.; Tan, L.; Zhang, C.; Cui, N.; Xiong, S.; Feng, J.; Qian, Y. Two-Dimensional Silicon/Carbon from Commercial Alloy and CO2 for Lithium Storage and Flexible Ti3C2Tx MXene-Based Lithium-Metal Batteries. ACS Nano 2020, 14, 17574–17588. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lu, M.; Han, W.; Li, H.; Wu, Y.; Zhang, W.; Wang, J.; Zhang, B. Employing MXene as a matrix for loading amorphous Si generated upon lithiation towards enhanced lithium-ion storage. J. Energy Chem. 2019, 38, 50–54. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Zhu, Q.; Guan, Z.; Zhao, Q.; Sun, N.; Xu, B. A Flexible Si@C Electrode with Excellent Stability Employing an MXene as a Multifunctional Binder for Lithium-Ion Batteries. ChemSusChem 2020, 13, 1621–1628. [Google Scholar] [CrossRef]
- Tian, Y.; An, Y.; Feng, J. Flexible and Freestanding Silicon/MXene Composite Papers for High-Performance Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11, 10004–10011. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Ren, M.; Xiong, J.; Pan, L.; Wang, Y.; Ji, X.; Qiu, T.; Yang, J.; Zhang, C. Self-assembly of hierarchical Ti3C2Tx-CNT/SiNPs resilient films for high performance lithium ion battery electrodes. Electrochim. Acta 2020, 348, 136211. [Google Scholar] [CrossRef]
- Hui, X.; Zhao, R.; Zhang, P.; Li, C.; Wang, C.; Yin, L. Low-Temperature Reduction Strategy Synthesized Si/Ti3C2 MXene Composite Anodes for High-Performance Li-Ion Batteries. Adv. Energy Mater. 2019, 9, 1901065. [Google Scholar] [CrossRef]
- Jo, D.Y.; Kim, J.K.; Oh, H.G.; Kang, Y.C.; Park, S.-K. Chemically Integrating MXene Nanosheets with N-Doped C-Coated Si Nanoparticles for Enhanced Li Storage Performance. Scr. Mater. 2021, 199, 113840. [Google Scholar] [CrossRef]
- Wang, Z.; Cao, D.; Ren, M.; Zhang, H.; Pan, L.; John Zhang, C.; Yang, J. Si@Ti3C2Tx with Si nanoparticles embedded in a 3D conductive network of crumpled Ti3C2Tx nanosheets for the anode of lithium-ion batteries with enhanced cycling performance. J. Alloys Compd. 2022, 892, 162037. [Google Scholar] [CrossRef]
- Li, X.; Chen, Z.; Li, A.; Yu, Y.; Chen, X.; Song, H. Three-Dimensional Hierarchical Porous Structures Constructed by Two-Stage MXene-Wrapped Si Nanoparticles for Li-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 48718–48728. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, X.; Yan, P.; Cheng, R.; Tang, Y.; Cui, M.; Wang, B.; Zhang, L.; Wang, X.; Jiang, Y.; et al. Dual Bond Enhanced Multidimensional Constructed Composite Silicon Anode for High-Performance Lithium Ion Batteries. ACS Nano 2019, 13, 8854–8864. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Zhang, F.; Zhu, G.; Ma, Y.; Luo, W.; Zhou, T.; Yang, J. Interface-Amorphized Ti3C2@Si/SiOx@TiO2 Anodes with Sandwiched Structures and Stable Lithium Storage. ACS Appl. Mater. Interfaces 2020, 12, 24796–24805. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Jiang, M.; Gao, H.; Chen, J.; Liu, W.; Ma, Y.; Luo, W.; Yang, J. Comparison of Additives in Anode: The Case of Graphene, MXene, CNTs Integration with Silicon Inside Carbon Nanofibers. Acta Metall. Sin. Engl. Lett. 2020, 34, 337–346. [Google Scholar] [CrossRef]
- Meng, J.; Zhang, F.; Zhang, L.; Liu, L.; Chen, J.; Yang, B.; Yan, X. Rolling up MXene sheets into scrolls to promote their anode performance in lithium-ion batteries. J. Energy Chem. 2020, 46, 256–263. [Google Scholar] [CrossRef]
- Xia, M.; Chen, B.; Gu, F.; Zu, L.; Xu, M.; Feng, Y.; Wang, Z.; Zhang, H.; Zhang, C.; Yang, J. Ti3C2Tx MXene Nanosheets as a Robust and Conductive Tight on Si Anodes Significantly Enhance Electrochemical Lithium Storage Performance. ACS Nano 2020, 14, 5111–5120. [Google Scholar] [CrossRef]
- Yan, Y.; Zhao, X.; Dou, H.; Wei, J.; Sun, Z.; He, Y.S.; Dong, Q.; Xu, H.; Yang, X. MXene Frameworks Promote the Growth and Stability of LiF-Rich Solid-Electrolyte Interphases on Silicon Nanoparticle Bundles. ACS Appl. Mater. Interfaces 2020, 12, 18541–18550. [Google Scholar] [CrossRef]
- Zhou, H.; Cui, C.; Cheng, R.; Yang, J.; Wang, X. MXene Enables Stable Solid-Electrolyte Interphase for Si@MXene Composite with Enhanced Cycling Stability. ChemElectroChem 2021, 8, 3089–3094. [Google Scholar] [CrossRef]
- Yan, Y.; He, Y.-S.; Zhao, X.; Zhao, W.; Ma, Z.-F.; Yang, X. Regulating adhesion of solid-electrolyte interphase to silicon via covalent bonding strategy towards high Coulombic-efficiency anodes. Nano Energy 2021, 84, 105935. [Google Scholar] [CrossRef]
- Mu, G.; Mu, D.; Wu, B.; Ma, C.; Bi, J.; Zhang, L.; Yang, H.; Wu, F. Microsphere-Like SiO2/MXene Hybrid Material Enabling High Performance Anode for Lithium Ion Batteries. Small 2020, 16, 1905430. [Google Scholar] [CrossRef]
- Mei, S.; Liu, Y.; Fu, J.; Guo, S.; Deng, J.; Peng, X.; Zhang, X.; Gao, B.; Huo, K.; Chu, P.K. Waste-glass-derived silicon/CNTs composite with strong Si-C covalent bonding for advanced anode materials in lithium-ion batteries. Appl. Surf. Sci. 2021, 563, 150280. [Google Scholar] [CrossRef]
- Tan, Y.; Jiang, T.; Chen, G.Z. Mechanisms and Product Options of Magnesiothermic Reduction of Silica to Silicon for Lithium-Ion Battery Applications. Front. Energy Res. 2021, 9, 651386. [Google Scholar] [CrossRef]
- Liang, M.; Wang, W.; Jiang, Y.; Liao, C.; Long, Q.; Lai, X.; Liao, L. Fabrication of C@Si@G for flexible lithium-ion batteries. J. Alloys Compd. 2021, 878, 160357. [Google Scholar] [CrossRef]
- Shao, J.; Yang, Y.; Zhang, X.; Shen, L.; Bao, N. 3D Yolk-Shell Structured Si/void/rGO Free-Standing Electrode for Lithium-Ion Battery. Materials 2021, 14, 2836. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Wang, M.; Li, Z.; Fan, G.; Fan, L.-Z.; Zhou, A. Two-dimensional Ti3C2 as anode material for Li-ion batteries. Electrochem. Commun. 2014, 47, 80–83. [Google Scholar] [CrossRef]
- Cheng, R.; Hu, T.; Zhang, H.; Wang, C.; Hu, M.; Yang, J.; Cui, C.; Guang, T.; Li, C.; Shi, C.; et al. Understanding the Lithium Storage Mechanism of Ti3C2Tx MXene. J. Phys. Chem. C 2018, 123, 1099–1109. [Google Scholar] [CrossRef] [Green Version]
- Das, P.; Wu, Z.-S. MXene for energy storage: Present status and future perspectives. J. Phys. Energy 2020, 2, 032004. [Google Scholar] [CrossRef]
- Wei, C.; Fei, H.; Tian, Y.; An, Y.; Tao, Y.; Li, Y.; Feng, J. Scalable construction of SiO/wrinkled MXene composite by a simple electrostatic self-assembly strategy as anode for high-energy lithium-ion batteries. Chin. Chem. Lett. 2020, 31, 980–983. [Google Scholar] [CrossRef]
- Kshetri, T.; Tran, D.T.; Le, H.T.; Nguyen, D.C.; Hoa, H.V.; Kim, N.H.; Lee, J.H. Recent advances in MXene-based nanocomposites for electrochemical energy storage applications. Prog. Mater. Sci. 2021, 117, 100733. [Google Scholar] [CrossRef]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.Q.; Gogotsi, Y.; Barsoum, M.W. Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance. Nature 2014, 516, 78–81. [Google Scholar] [CrossRef]












| Structure | Advantages | Disadvantages |
|---|---|---|
| Layer-by-Layer |
|
|
| Self-standing |
|
|
| 3D |
|
|
| Coating |
|
|
| Preparation Methods | Advantages | Disadvantages |
|---|---|---|
| Mechanical mixing |
|
|
| Wet processing |
|
|
| Spray drying |
|
|
| Magnesiothermic reduction |
|
|
| Filtration |
|
|
| Freeze-drying |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, T.; Yang, H.; Chen, G.Z. Enhanced Performance of Silicon Negative Electrodes Composited with Titanium Carbide Based MXenes for Lithium-Ion Batteries. Nanoenergy Adv. 2022, 2, 165-196. https://doi.org/10.3390/nanoenergyadv2020007
Jiang T, Yang H, Chen GZ. Enhanced Performance of Silicon Negative Electrodes Composited with Titanium Carbide Based MXenes for Lithium-Ion Batteries. Nanoenergy Advances. 2022; 2(2):165-196. https://doi.org/10.3390/nanoenergyadv2020007
Chicago/Turabian StyleJiang, Tingting, Hao Yang, and George Zheng Chen. 2022. "Enhanced Performance of Silicon Negative Electrodes Composited with Titanium Carbide Based MXenes for Lithium-Ion Batteries" Nanoenergy Advances 2, no. 2: 165-196. https://doi.org/10.3390/nanoenergyadv2020007