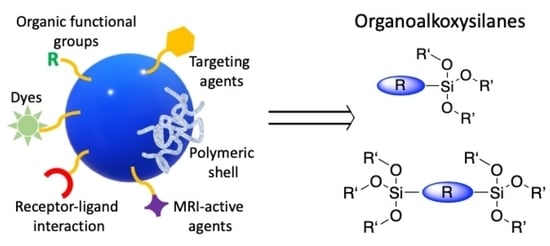
Synthesis of Organoalkoxysilanes: Versatile Organic–Inorganic Building Blocks
Abstract
:1. Introduction
2. Organoalkoxysilanes
3. Synthesis of Organoalkoxysilanes
3.1. Hydrosilylation
3.2. Isocyanates Addition Reactions
3.3. Nucleophilic Substitution Reactions
3.4. Nucleophilic Addition Reactions
3.5. Click Chemistry Tools
4. Direct Application of Commercial Organoalkoxysilanes in Silica-Based Materials
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Jaroniec, M. Silicon beyond the valley. Nat. Chem. 2009, 1, 166. [Google Scholar] [CrossRef] [PubMed]
- Gröger, C.; Lutz, K.; Brunner, E. Biomolecular Self-assembly and its Relevance in Silica Biomineralization. Cell Biochem. Biophys. 2008, 50, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Foo, C.W.P.; Huang, J.; Kaplan, D.L. Lessons from seashells: Silica mineralization via protein templating. TRENDS Biotechnol. 2004, 22, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Liberman, A.; Mendez, N.; Trogler, W.C.; Kummel, A.C. Synthesis and surface functionalization of silica nanoparticles for nanomedicine. Surf. Sci. Rep. 2014, 69, 132–158. [Google Scholar] [CrossRef] [Green Version]
- Croissant, J.G.; Butler, K.S.; Zink, J.I.; Brinker, C.F. Synthetic amorphous silica nanoparticles: Toxicity, biomedical and environmental implications. Nat. Rev. Mater. 2020, 5, 886–909. [Google Scholar] [CrossRef]
- Putro, W.S.; Lee, V.Y.; Sato, K.; Choi, J.-C.; Fukaya, N. From SiO2 to Alkoxysilanes for the Synthesis of Useful Chemicals. ACS Omega 2021, 6, 35186–35195. [Google Scholar] [CrossRef] [PubMed]
- Dankert, F.; von Hanisch, C. Siloxane Coordination Revisited: Si-O Bond Character, Reactivity and Magnificent Molecular Shapes. Eur. J. Inorg. Chem. 2021, 2021, 2907–2927. [Google Scholar] [CrossRef]
- Nayl, A.A.; Abd-Elhamid, A.I.; Aly, A.A.; Brase, S. Recent progress in the applications of silica-based nanoparticles. RSC Adv. 2022, 12, 13706–13726. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Srivastava, S.; Singh, S.K. Nanosilica: Recent Progress in Synthesis, Functionalization, Biocompatibility, and Biomedical Applications. ACS Biomater. Sci. Eng. 2019, 5, 4882–4898. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, M.C. Sol-Gel Silica Nanoparticles in Medicine: A Natural Choice. Design, Synthesis and Products. Molecules 2018, 23, 2021. [Google Scholar] [CrossRef] [Green Version]
- Akhter, F.; Rao, A.A.; Abbasi, M.N.; Wahocho, S.A.; Mallah, M.A.; Annes-ur-Rehman, H.; Chandio, Z.A. A Comprehensive Review of Synthesis, Applications and Future Prospects for Silica Nanoparticles (SNPs). Silicon 2022, 14, 8295–8310. [Google Scholar] [CrossRef]
- Issa, A.A.; Luyt, A.S. Kinetics of Alkoxysilanes and Organoalkoxysilanes Polymerization: A Review. Polymers 2019, 11, 537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manning, J.R.H.; Brambila, C.; Patwardhan, S.V. Unified mechanistic interpretation of amine-assisted silica synthesis methods to enable design of more complex materials. Mol. Syst. Des. Eng. 2021, 6, 170–196. [Google Scholar] [CrossRef]
- Carcouët, C.C.M.C.; van de Put, M.W.P.; Mezari, B.; Magusin, P.C.M.M.; Laven, J.; Bomans, P.H.H.; Friedrich, H.; Esteves, A.C.C.; Sommerdijk, N.A.J.M.; van Benthem, R.A.T.M.; et al. Nucleation and Growth of Monodisperse Silica Nanoparticles. Nano Lett. 2014, 14, 1433–1438. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, P.P.; Jaroniec, M. Renaissance of Stöber method for synthesis of colloidal particles: New developments and opportunities. J. Colloid Interface Sci. 2021, 584, 838–865. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, E.; Ansari, L.; Abnous, K.; Taghdisi, S.M.; Charbgoo, F.; Ramezani, M.; Alibolandi, M. Silica based hybrid materials for drug delivery and bioimaging. J. Contr. Rel. 2018, 277, 57–76. [Google Scholar] [CrossRef]
- Zhuravlev, L.T. The surface chemistry of amorphous silica. Zhuravlev model. Colloids Surf. A Physicochem. Eng. Asp. 2000, 173, 1–38. [Google Scholar] [CrossRef] [Green Version]
- Zuo, Y.; Liang, X.; Yin, J.; Gou, Z.; Lin, W. Understanding the significant role of SiAOASi bonds: Organosilicon materials as powerful platforms for bioimaging. Coord. Chem. Rev. 2021, 447, 214166. [Google Scholar] [CrossRef]
- Croissant, J.G.; Cattoën, X.; Durand, J.O.; Wong Chi Man, M.; Khashab, N.M. Organosilica hybrid nanomaterials with a high organic content: Syntheses and applications of silsesquioxanes. Nanoscale 2016, 8, 19945–19972. [Google Scholar] [CrossRef]
- Noureddine, A.; Brinker, C.F. Pendant/bridged/mesoporous silsesquioxane nanoparticles: Versatile and biocompatible platforms for smart delivery of therapeutics. J. Chem. Eng. 2018, 340, 125–147. [Google Scholar] [CrossRef]
- Rathnayake, H.; White, J.; Dawood, S. Polysilsesquioxane-Based Organic—Inorganic Hybrid Nanomaterials and Their Applications Towards Organic Photovoltaics. Synth. Met. 2021, 273, 116705. [Google Scholar] [CrossRef]
- Urata, C.; Yamada, H.; Wakabayashi, R.; Aoyama, Y.; Hirosawa, S.; Arai, S.; Takeoka, S.; Yamauchi, Y.; Kuroda, K. Aqueous Colloidal Mesoporous Nanoparticles with Ethenylene-Bridged Silsesquioxane Frameworks. J. Am. Chem. Soc. 2011, 133, 8102–8105. [Google Scholar] [CrossRef] [PubMed]
- Lipke, M.C.; Liberman-Martin, A.L.; Tilley, T.D. Electrophilic Activation of Silicon-Hydrogen Bonds in Catalytic Hydrosilations. Angew. Chem. 2016, 56, 2260–2294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofman, R.J.; Vlatković, M.; Wiesbrock, F. Fifty Years of Hydrosilylation in Polymer Science: A Review of Current Trends of Low-Cost Transition-Metal and Metal-Free Catalysts, Non-Thermally Triggered Hydrosilylation Reactions, and Industrial Applications. Polymers 2017, 9, 534. [Google Scholar] [CrossRef] [Green Version]
- Corma, A.; Botella, P.; Rivero-Buceta, E. Silica-Based Stimuli-Responsive Systems for Antitumor Drug Delivery and Controlled Release. Pharmaceutics 2022, 14, 110. [Google Scholar] [CrossRef]
- Corma, A.; Díaz, U.; Arrica, M.; Fernández, E.; Ortega, I. Organic–Inorganic Nanospheres with Responsive Molecular Gates for Drug Storage and Release. Angew. Chem. Int. Ed. 2009, 48, 6247–6250. [Google Scholar] [CrossRef]
- Picchetti, P.; DiMarco, B.N.; Travaglini, L.; Zhang, Y.; Ortega-Liebana, M.C.; De Cola, L. Breaking with Light: Stimuli-Responsive Mesoporous Organosilica Particles. Chem. Mater. 2020, 32, 392–399. [Google Scholar] [CrossRef]
- Zhao, L.; Loy, D.A.; Shea, K.J. Photodeformable Spherical Hybrid Nanoparticles. J. Am. Chem. Soc. 2006, 128, 14250–14251. [Google Scholar] [CrossRef]
- Crucho, C.I.C.; Barros, M.T. Sweet as sucrose. Nat. Chem. 2023, 15, 154. [Google Scholar] [CrossRef]
- Henkensmeier, D.; Abele, B.C.; Candussio, A.; Thiem, J. Synthesis and Characterisation of Terminal Carbohydrate Modified Poly(dimethylsiloxane)s. Macromol. Chem. Phys. 2004, 205, 1851–1857. [Google Scholar] [CrossRef]
- Buchan, Z.A.; Bader, S.J.; Montgomery, J. Ketone Hydrosilylation with Sugar Silanes Followed by Intramolecular Aglycone Delivery: An Orthogonal Glycosylation Strategy. Angew. Chem. Int. Ed. Engl. 2009, 48, 4840–4844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walk, J.T.; Buchan, Z.A.; Montgomery, J. Sugar silanes: Versatile reagents for stereocontrolled glycosylation via intramolecular aglycone delivery. Chem. Sci. 2015, 6, 3448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, X.; Zhang, X.; Yang, H.; Ji, X.; Li, J.; Ding, S. Iridium-Catalyzed Hydrosilylation of Unactivated Alkenes: Scope and Application to Late-Stage Functionalization. J. Org. Chem. 2019, 84, 1085–1093. [Google Scholar] [CrossRef]
- Ulanowska, M.; Olas, B. Biological Properties and Prospects for the Application of Eugenol—A Review. Int. J. Mol. Sci. 2021, 22, 3671. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Lu, Z.; Wang, J.; Shen, J.; Hao, Q.; Li, Y.; Yang, J.; Niu, Y.; Xiao, Z.; Chen, L. Preparation of mesoporous silica nanoparticle with tunable pore diameters for encapsulating and slowly releasing eugenol. Chin. Chem. Lett. 2021, 32, 1755–1758. [Google Scholar] [CrossRef]
- Shahabadi, N.; Akbari, A.; Karampour, F.; Falsafi, M. Cytotoxicity and antibacterial activities of new chemically synthesized magnetic nanoparticles containing eugenol. J. Drug Deliv. Sci. Technol. 2019, 49, 113–122. [Google Scholar] [CrossRef]
- Sokolnicki, T.; Franczyk, A.; Janowski, B.; Walkowiak, J. Synthesis of Bio-Based Silane Coupling Agents by the Modification of Eugenol. Adv. Synth. Catal. 2021, 363, 5493–5500. [Google Scholar] [CrossRef]
- Delebecq, E.; Pascault, J.-P.; Boutevin, B.; Ganachaud, F. On the Versatility of Urethane/Urea Bonds: Reversibility, Blocked Isocyanate, and Non-isocyanate Polyurethane. Chem. Rev. 2013, 113, 80–118. [Google Scholar] [CrossRef]
- Filho, J.G.O.; Egea, M.B. Edible Bioactive Film with Curcumin: A Potential “Functional” Packaging? Int. J. Mol. Sci. 2022, 23, 5638. [Google Scholar] [CrossRef]
- Karthikeyan, A.; Senthil, N.; Min, T. Nanocurcumin: A Promising Candidate for Therapeutic Applications. Front. Pharmacol. 2020, 11, 487. [Google Scholar] [CrossRef]
- Datz, S.; Engelke, H.; Schirnding, C.; Nguyen, L.; Bein, T. Lipid Bilayer-Coated Curcumin-based Mesoporous Organosilica Nanoparticles for Cellular Delivery. Microporous Mesoporous Mater. 2016, 225, 371–377. [Google Scholar] [CrossRef] [Green Version]
- Maggini, L.; Travaglini, L.; Cabrera, I.; Castro-Hartmann, P.; De Cola, L. Biodegradable Peptide–Silica Nanodonuts. Chem. Eur. J. 2016, 22, 3697–3703. [Google Scholar] [CrossRef] [PubMed]
- Lerouge, F.; Cerveau, G.; Corriu, R.J.P.; Stern, C.; Guilard, R. Self-organization of porphyrin units induced by magnetic field during sol–gel polymerization. Chem. Commun. 2007, 15, 1553–1555. [Google Scholar] [CrossRef] [PubMed]
- Fuerte, A.; Corma, A.; Iglesias, M.; Morales, E.; Sànchez, F. Approaches to the synthesis of heterogenised metalloporphyrins Application of new materials as electrocatalysts for oxygen reduction. J. Mol. Catal. A Chem. 2006, 246, 109–117. [Google Scholar] [CrossRef]
- Aggad, D.; Jimenez, C.M.; Dib, S.; Croissant, J.G.; Lichon, L.; Laurencin, D.; Richeter, S.; Maynadier, M.; Alsaiari, S.K.; Boufatit, M.; et al. Gemcitabine Delivery and Photodynamic Therapy in cancer cells via Porphyrin-Ethylene-Based Periodic Mesoporous Organosilica Nanoparticles. ChemNanoMat 2018, 4, 46–51. [Google Scholar] [CrossRef] [Green Version]
- Mahy, J.G.; Paez, C.A.; Carcel, C.; Bied, C.; Tatton, A.S.; Damblon, C.; Heinrichs, B.; Man, M.C.M.; Lambert, S.D. Porphyrin-, based hybrid silica-titania as visible-light photocatalyst. J. Photochem. Photobiol. A Chem. 2019, 373, 66–76. [Google Scholar] [CrossRef]
- Gao, Z.; Moghaddam, S.P.H.; Ghandehari, H.; Zharov, I. Synthesis of water-degradable silica nanoparticles from carbamate-containing bridged silsesquioxane precursor. RSC Adv. 2018, 8, 4914–4920. [Google Scholar] [CrossRef] [Green Version]
- Valot, L.; Maumus, M.; Montheil, T.; Martinez, J.; Noel, D.; Mehdi, A.; Subra, G. Biocompatible Glycine-Assisted Catalysis of the Sol-Gel Process: Development of Cell-Embedded Hydrogels. ChemPlusChem 2019, 84, 1720–1729. [Google Scholar] [CrossRef] [Green Version]
- Montheil, T.; Raimond, L.; Valot, L.; Maumus, M.; Simon, M.; Martinez, J.; Amblard, M.; Noel, D.; Mehdi, A.; Subra, G. Controlled Silylation of Polysaccharides: Attractive Building Blocks for Biocompatible Foams and Cell-Laden Hydrogels. ACS Appl. Polym. Mater. 2022, 4, 4087–4097. [Google Scholar] [CrossRef]
- Vivero-Escoto, J.L.; Rieter, W.J.; Lau, H.; Huxford-Phillips, R.C.; Lin, W. Biodegradable Polysilsesquioxane Nanoparticles as Efficient Contrast Agents for Magnetic Resonance Imaging. Small 2013, 9, 3523–3531. [Google Scholar] [CrossRef] [Green Version]
- Nagy, P. Kinetics and Mechanisms of Thiol–Disulfide Exchange Covering Direct Substitution and Thiol Oxidation-Mediated Pathway. Antioxid. Redox Signal. 2013, 18, 1623–1641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mollazadeh, S.; Mackiewicz, M.; Yazdimamaghani, M. Recent Advances in the Redox-Responsive Drug Delivery Nanoplatforms: A Chemical Structure and Physical Property Perspective. Mater. Sci. Eng. C 2021, 118, 111536. [Google Scholar] [CrossRef]
- Vimal, K.J. Chapter 1: An Overview of Organoselenium Chemistry: From Fundamentals to Synthesis. In Organoselenium Compounds in Biology and Medicine: Synthesis, Biological and Therapeutic Treatments; Royal Society of Chemistry: London, UK, 2017; pp. 1–33. [Google Scholar]
- Bisht, N.; Phalswal, P.; Khanna, P.K. Selenium nanoparticles: A review on synthesis and biomedical applications. Mater. Adv. 2022, 3, 1415–1431. [Google Scholar] [CrossRef]
- Shao, D.; Li, M.; Wang, Z.; Zheng, X.; Lao, Y.H.; Chang, Z.; Zhang, F.; Lu, M.; Yue, J.; Hu, H.; et al. Bioinspired Diselenide-Bridged Mesoporous Silica Nanoparticles for Dual-Responsive Protein Delivery. Adv. Mater. 2018, 30, 1801198. [Google Scholar] [CrossRef] [PubMed]
- Fatieiev, Y.; Croissant, J.G.; Julfakyan, K.; Deng, L.; Anjum, D.H.; Gurinov, A.; Khashab, N.M. Enzymatically degradable hybrid organic– inorganic bridged silsesquioxane nanoparticles for in vitro imaging. Nanoscale 2015, 7, 15046–15050. [Google Scholar] [CrossRef] [Green Version]
- Croissant, J.G.; Fatieiev, Y.; Julfakyan, K.; Lu, J.; Emwas, A.-H.; Anjum, D.H.; Omar, H.; Tamanoi, F.; Zink, J.I.; Khashab, N.M. Biodegradable Oxamide-Phenylene-Based Mesoporous Organosilica Nanoparticles with Unprecedented Drug Payloads for Delivery in Cells. Chem. Eur. J. 2016, 22, 14806–14811. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Kong, C.; Huo, M.; Liu, C.; Peng, L.; Zhao, T.; Wei, Y.; Qian, F.; Yuan, J. Schiff base interactions tuned mesoporous organosilica nanoplatforms with pH-responsive degradability for efficient anti-cancer drug delivery in vivo. Chem. Commun. 2018, 54, 9190–9193. [Google Scholar] [CrossRef] [PubMed]
- Travaglini, L.; Picchetti, P.; Totovao, R.; Prasetyanto, E.A.; De Cola, L. Highly degradable imine-doped mesoporous silica particles. Mater. Chem. Front. 2019, 3, 111–119. [Google Scholar] [CrossRef]
- Lima, M.; Avó, J.; Berberan-Santos, M.N.; Crucho, C.I.C. pH-responsive Silica Coatings: A Generic Approach for Smart Protection of Colloidal Nanoparticles. ACS Appl. Nano Mater. 2022, 5, 9460–9468. [Google Scholar] [CrossRef]
- Devaraj, N.K.; Finn, M.G. Introduction: Click Chemistry. Chem. Rev. 2021, 121, 6697–6698. [Google Scholar] [CrossRef]
- Croissant, J.G.; Mauriello-Jimenez, C.; Maynadier, M.; Cattoën, X.; Wong Chi Man, M.; Raehm, L.; Mongin, O.; Blanchard-Desce, M.; Garcia, M.; Gary-Bobo, M.; et al. Synthesis of Disulfide-Based Biodegradable Bridged Silsesquioxane Nanoparticles for Two-Photon Imaging and Therapy of Cancer Cells. Chem. Commun. 2015, 51, 12324–12327. [Google Scholar] [CrossRef]
- Croissant, J.; Maynadier, M.; Mongin, O.; Hugues, V.; Blanchard-Desce, M.; Chaix, A.; Cattoën, X.; Wong Chi Man, M.; Gallud, A.; Gary-Bobo, M.; et al. Enhanced Two-Photon Fluorescence Imaging and Therapy of Cancer Cells via Gold@Bridged Silsesquioxane Nanoparticles. Small 2015, 11, 295–299. [Google Scholar] [CrossRef]
- von Baeckmann, C.; Kahlig, H.; Lindén, M.; Kleitz, F. On the importance of the linking chemistry for the PEGylation of mesoporous silica nanoparticles. J. Colloid Interface Sci. 2021, 589, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Crucho, C.I.C.; Baleizão, C.; Farinha, J.P.S. Functional group coverage and conversion quantification in nanostructured silica by 1H-NMR. Anal. Chem. 2017, 89, 681–687. [Google Scholar] [CrossRef]
- Meng, H.; Xue, M.; Xia, T.; Zhao, Y.-L.; Tamanoi, F.; Stoddart, J.F.; Zink, J.I.; Nel, A.E. Autonomous in Vitro Anticancer Drug Release from Mesoporous Silica Nanoparticles by pH-Sensitive Nanovalves. J. Am. Chem. Soc. 2010, 132, 12690–12697. [Google Scholar] [CrossRef] [Green Version]
- Porta, F.; Lamers, G.E.M.; Morrhayim, J.; Chatzopoulou, A.; Schaaf, M.; den Dulk, H.; Backendorf, C.; Zink, J.I.; Kros, A. Folic Acid-Modified Mesoporous Silica Nanoparticles for Cellular and Nuclear Targeted Drug Delivery. Adv. Healthcare Mater. 2012, 2, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Quesada, M.; Muniesa, C.; Botella, P. Hybrid PLGA-Organosilica Nanoparticles with Redox-Sensitive Molecular Gates. Chem. Mater. 2013, 13, 2597–2602. [Google Scholar] [CrossRef]
- Croissant, J.; Cattoën, X.; Wong Chi Man, M.; Gallud, A.; Raehm, L.; Trens, P.; Maynadier, M.; Durand, J.-O. Biodegradable Ethylene-Bis(Propyl)Disulfide-Based Periodic Mesoporous Organosilica Nanorods and Nanospheres for Efficient In-Vitro Drug Delivery. Adv. Mater. 2014, 26, 6174–6180. [Google Scholar] [CrossRef]
- Maggini, L.; Cabrera, I.; Ruiz-Carretero, A.; Prasetyanto, E.A.; Robinet, E.; De Cola, L. Breakable mesoporous silica nanoparticles for targeted drug delivery. Nanoscale 2016, 8, 7240. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, W.; Su, B.; Wen, Y.; Li, C.; Zhou, Y.; Li, M.; Shi, X.; Du, H.; Song, Y.; et al. A light-responsive release platform by controlling the wetting behaviour of hydrophobic surface. ACS Nano 2014, 8, 744–751. [Google Scholar] [CrossRef]
- Rivero-Buceta, E.; Vidaurre-Agut, C.; Vera-Donoso, C.D.; Benlloch, J.M.; Moreno-Manzano, V.; Botella, P. PSMA-Targeted Mesoporous Silica Nanoparticles for Selective Intracellular Delivery of Docetaxel in Prostate Cancer Cells. ACS Omega 2019, 4, 1281–1291. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Bei, H.P.; Piao, Y.; Wang, Y.; Yang, M.; Zhao, X. Polymer-brush-grafted mesoporous silica nanoparticles for triggered drug delivery. ChemPhysChem 2018, 19, 1956–1964. [Google Scholar] [CrossRef] [Green Version]
- Hore, M.J.A. Polymers on nanoparticles: Structure & dynamics. Soft Matter 2019, 15, 1120–1134. [Google Scholar]
- Balis, A.; Wolski, K.; Zapotoczny, S. Thermoresponsive polymer gating system on mesoporous shells of silica particles serving as smart nanocontainers. Polymers 2020, 12, 888. [Google Scholar] [CrossRef] [PubMed]
- Raj, R.; Pinto, S.N.; Crucho, C.I.C.; Das, S.; Baleizão, C.; Farinha, J.P.S. Optically traceable PLGA-Silica Nanoparticles for Cell-Triggered Doxorubicin Delivery. Colloids Surf. B 2022, 220, 112872. [Google Scholar] [CrossRef] [PubMed]
- Quignard, S.; Masse, S.; Laurent, G.; Coradin, T. Introduction of disulfide bridges within silica nanoparticles to control their intra-cellular degradation. Chem. Commun. 2013, 49, 3410–3412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montheil, T.; Simon, M.; Noel, D.; Mehdi, A.; Subra, G.; Echalier, C. Silylated biomolecules: Versatile components for bioinks. Front. Bioeng. Biotechnol. 2022, 10, 888437. [Google Scholar] [CrossRef]

























Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crucho, C.I.C. Synthesis of Organoalkoxysilanes: Versatile Organic–Inorganic Building Blocks. Compounds 2023, 3, 280-297. https://doi.org/10.3390/compounds3010021
Crucho CIC. Synthesis of Organoalkoxysilanes: Versatile Organic–Inorganic Building Blocks. Compounds. 2023; 3(1):280-297. https://doi.org/10.3390/compounds3010021
Chicago/Turabian StyleCrucho, Carina I. C. 2023. "Synthesis of Organoalkoxysilanes: Versatile Organic–Inorganic Building Blocks" Compounds 3, no. 1: 280-297. https://doi.org/10.3390/compounds3010021