Nitric Oxide Production from Nitrite plus Ascorbate during Ischemia upon Hippocampal Glutamate NMDA Receptor Stimulation
Abstract
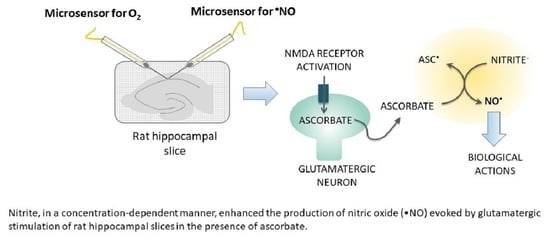
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Electrochemical Instrumentation
2.3. Carbon Fiber Microelectrode Fabrication and Surface Modification
2.4. Carbon Fiber Microelectrodes Calibration
2.5. Rat Hippocampal Slices
2.6. Nitric Oxide and Oxygen Recording
2.7. Data Analysis
3. Results
3.1. Nitric Oxide Concentration Dynamics in Rat Hippocampus Slices Evoked by NMDA Stimulation of Neuronal Activity: The Modulatory Role of Nitrite
3.2. Nitric Oxide Concentration Dynamics in Rat Hippocampus Slices upon Stimulation of Neuronal Activity by Glutamate
3.3. Evaluation of pH and Nitric Oxide Production in Rat Hippocampus Slices in Transient Oxygen-Glucose Deprivation (OGD)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Magistretti, P.J.; Pellerin, L.; Rothman, D.L.; Shulman, R.G. Energy on demand. Science 1999, 283, 496–497. [Google Scholar] [CrossRef]
- Jackman, K.; Iadecola, C. Neurovascular regulation in the ischemic brain. Antioxid. Redox Signal. 2015, 22, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Drake, C.T.; Iadecola, C. The role of neuronal signaling in controlling cerebral blood flow. Brain Lang. 2007, 102, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Hossmann, K.A. Viability thresholds and the penumbra of focal ischemia. Ann. Neurol. 1994, 36, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C. The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease. Neuron 2017, 96, 17–42. [Google Scholar] [CrossRef]
- Lourenco, C.F.; Laranjinha, J. Nitric Oxide Pathways in Neurovascular Coupling Under Normal and Stress Conditions in the Brain: Strategies to Rescue Aberrant Coupling and Improve Cerebral Blood Flow. Front. Physiol. 2021, 12, 729201. [Google Scholar] [CrossRef]
- Lourenco, C.F.; Santos, R.M.; Barbosa, R.M.; Cadenas, E.; Radi, R.; Laranjinha, J. Neurovascular coupling in hippocampus is mediated via diffusion by neuronal-derived nitric oxide. Free Radic. Biol. Med. 2014, 73, 421–429. [Google Scholar] [CrossRef]
- Hosford, P.S.; Gourine, A.V. What is the key mediator of the neurovascular coupling response? Neurosci. Biobehav. Rev. 2019, 96, 174–181. [Google Scholar] [CrossRef]
- Calabrese, V.; Mancuso, C.; Calvani, M.; Rizzarelli, E.; Butterfield, D.A.; Stella, A.M. Nitric oxide in the central nervous system: Neuroprotection versus neurotoxicity. Nat. Rev. Neurosci. 2007, 8, 766–775. [Google Scholar] [CrossRef]
- Laranjinha, J.; Nunes, C.; Ledo, A.; Lourenco, C.; Rocha, B.; Barbosa, R.M. The Peculiar Facets of Nitric Oxide as a Cellular Messenger: From Disease-Associated Signaling to the Regulation of Brain Bioenergetics and Neurovascular Coupling. Neurochem. Res. 2021, 46, 64–76. [Google Scholar] [CrossRef]
- Laranjinha, J.; Santos, R.M.; Lourenco, C.F.; Ledo, A.; Barbosa, R.M. Nitric oxide signaling in the brain: Translation of dynamics into respiration control and neurovascular coupling. Ann. N. Y. Acad. Sci. 2012, 1259, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Picon-Pages, P.; Garcia-Buendia, J.; Munoz, F.J. Functions and dysfunctions of nitric oxide in brain. Biochim. Et Biophys. Acta Mol. Basis Dis. 2019, 1865, 1949–1967. [Google Scholar] [CrossRef]
- Ledo, A.; Lourenco, C.F.; Cadenas, E.; Barbosa, R.M.; Laranjinha, J. The bioactivity of neuronal-derived nitric oxide in aging and neurodegeneration: Switching signaling to degeneration. Free Radic. Biol. Med. 2021, 162, 500–513. [Google Scholar] [CrossRef] [PubMed]
- Alderton, W.K.; Cooper, C.E.; Knowles, R.G. Nitric oxide synthases: Structure, function and inhibition. Biochem. J. 2001, 357, 593–615. [Google Scholar] [CrossRef]
- Moncada, S.; Palmer, R.M.; Higgs, E.A. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharm. Rev. 1991, 43, 109–142. [Google Scholar]
- Ledo, A.; Barbosa, R.M.; Gerhardt, G.A.; Cadenas, E.; Laranjinha, J. Concentration dynamics of nitric oxide in rat hippocampal subregions evoked by stimulation of the NMDA glutamate receptor. Proc. Natl. Acad. Sci. USA 2005, 102, 17483–17488. [Google Scholar] [CrossRef] [PubMed]
- Christopherson, K.S.; Hillier, B.J.; Lim, W.A.; Bredt, D.S. PSD-95 assembles a ternary complex with the N-methyl-D-aspartic acid receptor and a bivalent neuronal NO synthase PDZ domain. J. Biol. Chem. 1999, 274, 27467–27473. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–167. [Google Scholar] [CrossRef]
- Shiva, S. Nitrite: A Physiological Store of Nitric Oxide and Modulator of Mitochondrial Function. Redox Biol. 2013, 1, 40–44. [Google Scholar] [CrossRef]
- Millar, J. The nitric oxide/ascorbate cycle: How neurones may control their own oxygen supply. Med. Hypotheses 1995, 45, 21–26. [Google Scholar] [CrossRef]
- Kapil, V.; Khambata, R.S.; Jones, D.A.; Rathod, K.; Primus, C.; Massimo, G.; Fukuto, J.M.; Ahluwalia, A. The Noncanonical Pathway for In Vivo Nitric Oxide Generation: The Nitrate-Nitrite-Nitric Oxide Pathway. Pharm. Rev. 2020, 72, 692–766. [Google Scholar] [CrossRef]
- Gago, B.; Lundberg, J.O.; Barbosa, R.M.; Laranjinha, J. Red wine-dependent reduction of nitrite to nitric oxide in the stomach. Free Radic. Biol. Med. 2007, 43, 1233–1242. [Google Scholar] [CrossRef] [PubMed]
- Rocha, B.S.; Gago, B.; Barbosa, R.M.; Laranjinha, J. Dietary polyphenols generate nitric oxide from nitrite in the stomach and induce smooth muscle relaxation. Toxicology 2009, 265, 41–48. [Google Scholar] [CrossRef]
- Rifkind, J.M.; Nagababu, E.; Barbiro-Michaely, E.; Ramasamy, S.; Pluta, R.M.; Mayevsky, A. Nitrite infusion increases cerebral blood flow and decreases mean arterial blood pressure in rats: A role for red cell NO. Nitric Oxide 2007, 16, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Piknova, B.; Kocharyan, A.; Schechter, A.N.; Silva, A.C. The role of nitrite in neurovascular coupling. Brain Res. 2011, 1407, 62–68. [Google Scholar] [CrossRef]
- Brkic, D.; Bosnir, J.; Bevardi, M.; Boskovic, A.G.; Milos, S.; Lasic, D.; Krivohlavek, A.; Racz, A.; Cuic, A.M.; Trstenjak, N.U. Nitrate in Leafy Green Vegetables and Estimated Intake. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, M.; Kasahara, E.; Park, A.M.; Hiramoto, K.; Minamiyama, Y.; Takemura, S.; Sato, E.F.; Inoue, M. Dietary nitrate inhibits stress-induced gastric mucosal injury in the rat. Free Radic. Res. 2003, 37, 85–90. [Google Scholar] [CrossRef]
- Witter, J.P.; Balish, E.; Gatley, S.J. Distribution of nitrogen-13 from labeled nitrate and nitrite in germfree and conventional-flora rats. Appl. Env. Microbiol. 1979, 38, 870–878. [Google Scholar] [CrossRef]
- Duncan, C.; Dougall, H.; Johnston, P.; Green, S.; Brogan, R.; Leifert, C.; Smith, L.; Golden, M.; Benjamin, N. Chemical generation of nitric oxide in the mouth from the enterosalivary circulation of dietary nitrate. Nat. Med. 1995, 1, 546–551. [Google Scholar] [CrossRef]
- Benjamin, N.; O’Driscoll, F.; Dougall, H.; Duncan, C.; Smith, L.; Golden, M.; McKenzie, H. Stomach NO synthesis. Nature 1994, 368, 502. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E.; Lundberg, J.M.; Alving, K. Intragastric nitric oxide production in humans: Measurements in expelled air. Gut 1994, 35, 1543–1546. [Google Scholar] [CrossRef] [PubMed]
- Pluta, R.M.; Dejam, A.; Grimes, G.; Gladwin, M.T.; Oldfield, E.H. Nitrite infusions to prevent delayed cerebral vasospasm in a primate model of subarachnoid hemorrhage. Jama 2005, 293, 1477–1484. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, N.R.; Lourenco, C.F.; Barbosa, R.M.; Laranjinha, J. Coupling of ascorbate and nitric oxide dynamics in vivo in the rat hippocampus upon glutamatergic neuronal stimulation: A novel functional interplay. Brain Res. Bull. 2015, 114, 13–19. [Google Scholar] [CrossRef]
- Presley, T.D.; Morgan, A.R.; Bechtold, E.; Clodfelter, W.; Dove, R.W.; Jennings, J.M.; Kraft, R.A.; King, S.B.; Laurienti, P.J.; Rejeski, W.J.; et al. Acute effect of a high nitrate diet on brain perfusion in older adults. Nitric Oxide 2011, 24, 34–42. [Google Scholar] [CrossRef]
- Santos, R.M.; Lourenco, C.F.; Piedade, A.P.; Andrews, R.; Pomerleau, F.; Huettl, P.; Gerhardt, G.A.; Laranjinha, J.; Barbosa, R.M. A comparative study of carbon fiber-based microelectrodes for the measurement of nitric oxide in brain tissue. Biosens. Bioelectron. 2008, 24, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, G.A.; Hoffman, A.F. Effects of recording media composition on the responses of Nafion-coated carbon fiber microelectrodes measured using high-speed chronoamperometry. J. Neurosci. Methods 2001, 109, 13–21. [Google Scholar] [CrossRef]
- Ferreira, N.R.; Santos, R.M.; Laranjinha, J.; Barbosa, R.M. Real Time In Vivo Measurement of Ascorbate in the Brain Using Carbon Nanotube-Modified Microelectrodes. Electroanalysis 2013, 25, 1757–1763. [Google Scholar] [CrossRef]
- Cosby, K.; Partovi, K.S.; Crawford, J.H.; Patel, R.P.; Reiter, C.D.; Martyr, S.; Yang, B.K.; Waclawiw, M.A.; Zalos, G.; Xu, X.; et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat. Med. 2003, 9, 1498–1505. [Google Scholar] [CrossRef]
- Shiva, S.; Huang, Z.; Grubina, R.; Sun, J.; Ringwood, L.A.; MacArthur, P.H.; Xu, X.; Murphy, E.; Darley-Usmar, V.M.; Gladwin, M.T. Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circ. Res. 2007, 100, 654–661. [Google Scholar] [CrossRef]
- van Faassen, E.E.; Bahrami, S.; Feelisch, M.; Hogg, N.; Kelm, M.; Kim-Shapiro, D.B.; Kozlov, A.V.; Li, H.; Lundberg, J.O.; Mason, R.; et al. Nitrite as regulator of hypoxic signaling in mammalian physiology. Med. Res. Rev. 2009, 29, 683–741. [Google Scholar] [CrossRef]
- Shiva, S.; Sack, M.N.; Greer, J.J.; Duranski, M.; Ringwood, L.A.; Burwell, L.; Wang, X.; MacArthur, P.H.; Shoja, A.; Raghavachari, N.; et al. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J. Exp. Med. 2007, 204, 2089–2102. [Google Scholar] [CrossRef]
- Mefford, I.N.; Oke, A.F.; Adams, R.N. Regional distribution of ascorbate in human brain. Brain Res. 1981, 212, 223–226. [Google Scholar] [CrossRef]
- Milby, K.; Oke, A.; Adams, R.N. Detailed mapping of ascorbate distribution in rat brain. Neurosci. Lett. 1982, 28, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Rice, M.E.; Russo-Menna, I. Differential compartmentalization of brain ascorbate and glutathione between neurons and glia. Neuroscience 1998, 82, 1213–1223. [Google Scholar] [CrossRef] [PubMed]
- Miele, M.; Boutelle, M.G.; Fillenz, M. The physiologically induced release of ascorbate in rat brain is dependent on impulse traffic, calcium influx and glutamate uptake. Neuroscience 1994, 62, 87–91. [Google Scholar] [CrossRef]
- Sandstrom, M.I.; Rebec, G.V. Extracellular ascorbate modulates glutamate dynamics: Role of behavioral activation. BMC Neurosci. 2007, 8, 32. [Google Scholar] [CrossRef]
- Cammack, J.; Ghasemzadeh, B.; Adams, R.N. The pharmacological profile of glutamate-evoked ascorbic acid efflux measured by in vivo electrochemistry. Brain Res. 1991, 565, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Shaw, K.; Bell, L.; Boyd, K.; Grijseels, D.M.; Clarke, D.; Bonnar, O.; Crombag, H.S.; Hall, C.N. Neurovascular coupling and oxygenation are decreased in hippocampus compared to neocortex because of microvascular differences. Nat. Commun. 2021, 12, 3190. [Google Scholar] [CrossRef]
- Zhang, H.; Roman, R.J.; Fan, F. Hippocampus is more susceptible to hypoxic injury: Has the Rosetta Stone of regional variation in neurovascular coupling been deciphered? GeroScience 2022, 44, 127–130. [Google Scholar] [CrossRef]
- Ledo, A.; Barbosa, R.; Cadenas, E.; Laranjinha, J. Dynamic and interacting profiles of *NO and O2 in rat hippocampal slices. Free Radic. Biol. Med. 2010, 48, 1044–1050. [Google Scholar] [CrossRef]
- Park, S.S.; Hong, M.; Song, C.K.; Jhon, G.J.; Lee, Y.; Suh, M. Real-time in vivo simultaneous measurements of nitric oxide and oxygen using an amperometric dual microsensor. Anal. Chem. 2010, 82, 7618–7624. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Weitzberg, E. Nitrite reduction to nitric oxide in the vasculature. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H477–H478. [Google Scholar] [CrossRef] [PubMed]
- Funahashi, T.; Floyd, R.A.; Carney, J.M. Age effect on brain pH during ischemia/reperfusion and pH influence on peroxidation. Neurobiol. Aging 1994, 15, 161–167. [Google Scholar] [CrossRef]
- Nemoto, E.M.; Frinak, S. Brain tissue pH after global brain ischemia and barbiturate loading in rats. Stroke 1981, 12, 77–82. [Google Scholar] [CrossRef]
- von Hanwehr, R.; Smith, M.L.; Siesjo, B.K. Extra- and intracellular pH during near-complete forebrain ischemia in the rat. J. Neurochem. 1986, 46, 331–339. [Google Scholar] [CrossRef]
- Jung, K.H.; Chu, K.; Ko, S.Y.; Lee, S.T.; Sinn, D.I.; Park, D.K.; Kim, J.M.; Song, E.C.; Kim, M.; Roh, J.K. Early intravenous infusion of sodium nitrite protects brain against in vivo ischemia-reperfusion injury. Stroke 2006, 37, 2744–2750. [Google Scholar] [CrossRef] [PubMed]
- Dias, C.; Lourenco, C.F.; Laranjinha, J.; Ledo, A. Modulation of oxidative neurometabolism in ischemia/reperfusion by nitrite. Free Radic. Biol. Med. 2022, 193, 779–786. [Google Scholar] [CrossRef]
- Luettich, A.; Franko, E.; Spronk, D.B.; Lamb, C.; Corkill, R.; Patel, J.; Ezra, M.; Pattinson, K.T.S. Beneficial Effect of Sodium Nitrite on EEG Ischaemic Markers in Patients with Subarachnoid Haemorrhage. Transl. Stroke Res. 2022, 13, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Gladwin, M.T.; Kim-Shapiro, D.B. The functional nitrite reductase activity of the heme-globins. Blood 2008, 112, 2636–2647. [Google Scholar] [CrossRef]
- Cantu-Medellin, N.; Kelley, E.E. Xanthine oxidoreductase-catalyzed reduction of nitrite to nitric oxide: Insights regarding where, when and how. Nitric Oxide 2013, 34, 19–26. [Google Scholar] [CrossRef]
- Godber, B.L.; Doel, J.J.; Sapkota, G.P.; Blake, D.R.; Stevens, C.R.; Eisenthal, R.; Harrison, R. Reduction of nitrite to nitric oxide catalyzed by xanthine oxidoreductase. J. Biol. Chem. 2000, 275, 7757–7763. [Google Scholar] [CrossRef] [PubMed]
- Castello, P.R.; David, P.S.; McClure, T.; Crook, Z.; Poyton, R.O. Mitochondrial cytochrome oxidase produces nitric oxide under hypoxic conditions: Implications for oxygen sensing and hypoxic signaling in eukaryotes. Cell Metab. 2006, 3, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, A.V.; Staniek, K.; Nohl, H. Nitrite reductase activity is a novel function of mammalian mitochondria. FEBS Lett. 1999, 454, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Nohl, H.; Staniek, K.; Sobhian, B.; Bahrami, S.; Redl, H.; Kozlov, A.V. Mitochondria recycle nitrite back to the bioregulator nitric monoxide. Acta Biochim. Pol. 2000, 47, 913–921. [Google Scholar] [CrossRef]
- Vanin, A.F.; Bevers, L.M.; Slama-Schwok, A.; van Faassen, E.E. Nitric oxide synthase reduces nitrite to NO under anoxia. Cell. Mol. Life Sci. 2007, 64, 96–103. [Google Scholar] [CrossRef]
- Lourenco, C.F.; Ledo, A.; Barbosa, R.M.; Laranjinha, J. Neurovascular uncoupling in the triple transgenic model of Alzheimer’s disease: Impaired cerebral blood flow response to neuronal-derived nitric oxide signaling. Exp. Neurol. 2017, 291, 36–43. [Google Scholar] [CrossRef]
- Goncalves, J.S.; Seica, R.M.; Laranjinha, J.; Lourenco, C.F. Impairment of neurovascular coupling in the hippocampus due to decreased nitric oxide bioavailability supports early cognitive dysfunction in type 2 diabetic rats. Free Radic. Biol. Med. 2022, 193, 669–675. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, C.; Laranjinha, J. Nitric Oxide Production from Nitrite plus Ascorbate during Ischemia upon Hippocampal Glutamate NMDA Receptor Stimulation. BioChem 2023, 3, 78-90. https://doi.org/10.3390/biochem3020006
Nunes C, Laranjinha J. Nitric Oxide Production from Nitrite plus Ascorbate during Ischemia upon Hippocampal Glutamate NMDA Receptor Stimulation. BioChem. 2023; 3(2):78-90. https://doi.org/10.3390/biochem3020006
Chicago/Turabian StyleNunes, Carla, and João Laranjinha. 2023. "Nitric Oxide Production from Nitrite plus Ascorbate during Ischemia upon Hippocampal Glutamate NMDA Receptor Stimulation" BioChem 3, no. 2: 78-90. https://doi.org/10.3390/biochem3020006