1. Introduction
Melanin is a class of heterogeneous functional polymeric compounds produced by species from various kingdoms, including bacteria, fungi, animals, and plants. It is a biomacromolecule with a high molecular weight (318.3 g/mol) that is produced by the oxidative polymerization of phenolic or indolic molecules with a negatively charged hydrophobic polymer that is insoluble in both water and chemical solvents [
1,
2]. Melanin is an effective metal ion chelator, free radical scavenger, and absorber of UV–visible and visible light radiation. Melanin compounds have also been widely exploited for the development of innovative adhesive biomaterials and green bioelectronics due to their hybrid ionic–electronic conductance and redox reversibility features [
3]. Additionally, melanin has been employed to create novel, biodegradable, and biocompatible medical devices; nanoparticles; antibacterial drugs; antiviral and anticancer medicines; and radio-protective and antioxidant compounds. This is because they have excellent biocompatibility and biostability, are not cytotoxic, and do not provoke antigenic reactions. Due to melanin’s many beneficial properties, it has a wide range of applications in the agricultural, cosmetics, and medical industries [
4].
Currently, melanin has been mostly obtained from marine sources, for instance, the ink sac of the cuttlefish
Sepia officinalis. However, these approaches are often expensive, unsustainable, and highly dependent on the supply of such substances [
5]. On the other hand, it is generally agreed that the biotechnological methods of producing melanin from microorganisms are more scalable and environmentally friendly [
6]. Numerous bacteria, including
Streptomyces glaucescens,
Nocardiopsis alba, and
Pseudomonas stutzeri, and fungi, including
Armillaria ostoyae,
Aspergillus fumigatus, and
Daldinia concentrica, have been reported to produce melanin. The duration of microbial melanin production in culture can range from a few days to several weeks and is influenced by factors such as the type of microorganism, growth conditions, and the production method. The yield of melanin can vary greatly, ranging from milligrams per liter to grams per liter, depending on the strain and growth conditions [
6,
7].
Streptomyces strains produce melanins as primary pigments during the secondary metabolism phase [
8], but so far, melanin production by these bacteria has only been documented in a few research studies due to the lengthy processing times required, which can take anywhere between 120 and 168 h. The reported melanin yields range from 0.12 to 5.30 g/L according to the different culture conditions [
9,
10,
11,
12,
13,
14]. High productivity is needed for the feasible, large-scale application of biomaterials, including melanin. However, the amount of the substrate (L-tyrosine or L-DOPA) in the growth media used for tyrosinase determines the yield of melanin production [
15] from different bacteria, such as the soil bacterial isolates
Bacillus safensis [
16] and
Brevundimonas sp. SGJ [
17], and some fungi, such as
Auricularia auricula [
18] and
Aspergillus nidulans [
19]. Therefore, increasing melanin production efficiency can be accomplished via the natural selection of active enzymes or growth optimization.
Cyanobacteria, or blue-green algae, have recently risen to prominence in microbial research due to their ability to grow in non-potable water without competing with food crops for fertile soil and their contribution to reducing greenhouse gases in the environment through photosynthesis. Additionally, cyanobacterial biomass can be transformed into various high-value products, including biofuels, cosmetics, foods, nutraceuticals, and pharmaceutical compounds [
20].
A. platensis is one of the most important cyanobacteria genera and is well known for being a high source of protein (up to 50–70% of its cell mass) and bioactive ingredients such as vitamins, β-carotene, micro- and macro-elements, and polyunsaturated fatty acids [
21,
22]. Phycocyanin (PC) is a mainly water-soluble pigment protein (8–13% of dry biomass) that is found in cyanobacteria, particularly in
A. platensis [
23]. PC sparked attention because of its exceptional capability to exhibit antioxidant, anti-inflammatory, and anticarcinogenic properties. Furthermore, research has shown that it possesses anti-mutative, anti-microbial, antitumor, and wound-healing properties, and it has found applications as food coloring, in cosmetics, and as a dietary supplement [
24,
25].
The residue biomass left over after PC extraction from
A. platensis is still rich in valuable compounds such as proteins, carbohydrates, and lipids. This residue can be used for various purposes, including as livestock feed, fertilizer, or feedstock for biofuel production. Furthermore, some studies have investigated the production of crude bio-oil from biomass residues, and it has been discovered that nitrogen reduction via the elimination of pigment protein (PC) can considerably increase overall hydrothermal liquefaction efficiency [
26]. In earlier investigations, authors suggested recycling and transforming PC-extracted
Arthrospira sp. residue into porous, N-doped graphitic charcoal catalysts to construct an integrated oxidation system for wastewater treatment that would simultaneously remove contaminants and disinfect a given system [
27].
Protein hydrolysates are a complex mixture obtained from various protein sources (such as plants and animals) through various techniques, including chemical hydrolysis, microbial fermentation, and enzymatic hydrolysis [
28]. With considerable levels of amino acids, macro- and micro-elements, vitamins, and other organic substances, these protein hydrolysates can promote bacterial and fungal growth [
21,
29,
30]. The production of a range of microbial bioactive compounds using residual hydrolysates as low-cost substrates for culture media offers an affordable and sustainable production process.
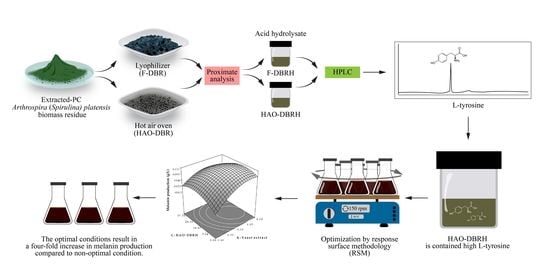
Our study presents a new and innovative method for producing melanin using S. antibioticus NRRL B-1701. We utilize HAO-DBRH as a cost-effective source of L-tyrosine, which is optimized using RSM. Importantly, this approach has not been previously reported in the literature.
4. Discussion
The PC-extracted residue of
A. platensis in the study has a dark-green and blue hue due to the remaining PC, as seen in
Figure 1A. Drying was used to remove water from solids and thus reduce microbial growth and chemical degradation [
39]. The drying methods used in this experiment were lyophilization (freeze drying) and drying via a hot air oven. Lyophilization dehydrates frozen samples by exposing them to low pressure and a temperature of −50 °C, causing ice crystals to sublimate. This method is commonly used for heat-sensitive materials [
40], such as PC, a pigment protein with a stability temperature of less than 45 °C [
31,
41]. A significant amount of PC’s blue color remained after drying using this method, as shown in
Figure 1B. The blue color observed in the samples is attributed to the presence of PC, which is a protein pigment found in cyanobacteria. PC is known to be sensitive to high temperatures [
42], which can lead to its denaturation. The application of the hot-air-oven-drying method, which involves a temperature of 60 °C, resulted in a loss of the blue color associated with PC, as illustrated in
Figure 1C.
The proximate analysis of F-DBR and HAO-DBR was carried out to determine the weight percentage and moisture content of various components such as ash, lipids, proteins, and carbohydrates (
Table 2). Both samples showed similar results in terms of moisture content, ash, lipids, and carbohydrates, which were averages of 5.88, 9.56, 5.17, and 12.5, respectively. These findings were consistent with previous reports in the literature [
43], which reported moisture content and ash, lipid, and carbohydrate concentrations in the range of 4–7, 6–12, 4–7, and 15–25, respectively.
Protein was the main constituent in both samples, accounting for 69.1% and 68.7% of the total dried biomass in F-DBR and HAO-DBR, respectively. This observation was higher than a previous report that found a protein fraction of 65.6 ± 0.12% in
A. platensis [
44]. The protein content found in this study is also in line with other studies that reported values in the range of 60–70% [
45,
46,
47]. Notably, protein content can vary depending on the algal growth conditions [
44]. Despite the removal of PC from the algal biomass, it was discovered that this residue is an excellent source of protein. However, the current work shows a comparable protein concentration of
Arthrospira sp. that was used for acid hydrolysis.
This study investigated the effect of drying methods on the residue of
A. platensis after PC extraction and acid hydrolysis. HPLC-UV was used for the quantitative analysis of L-tyrosine. Various techniques are available for amino acid quantification, such as ion-exchange chromatography, gas chromatography (GC), capillary electrophoresis (CE), mass spectrometry (MS), and high-performance liquid chromatography (HPLC). The selection of a method depends on specific analytical requirements, as each technique has its own advantages and disadvantages. HPLC is the most commonly used method for amino acid quantification due to its high sensitivity, specificity, and ability to separate amino acids in complex matrices. For example, Eid et al. [
48] used HPLC-UV for the simultaneous quantification of multiple underivatized amino acids in dietary supplements, including isoleucine, leucine, lysine, threonine, histidine, valine, methionine, phenylalanine, tryptophan, and tyrosine. Thus, HPLC is a suitable method for L-tyrosine quantification.
The minimum r
2 value requirement varies based on the field and purpose of research. The analytical method validation (AMV) protocols we have employed and read about require a minimum correlation coefficient of 0.990 for HPLC-UV [
49] and 0.950 for a spectrophotometer [
50]. Based on our results, r
2 = 0.997 is quite acceptable. The findings from the HPLC analysis showed that the L-tyrosine content in the hot-air-oven-dried biomass residue hydrolysate (HAO-DBRH) was higher compared to the freeze-dried biomass residue hydrolysate (F-DBRH). The higher L-tyrosine content in the HAO-DBRH was attributed to the higher temperature used in the hot-air-oven-drying process, which can result in the denaturation of proteins and the release of individual amino acids [
51]. Additionally, the denaturation of proteins can cause differences in amino acid profiles, as some amino acids may be more susceptible to hydrolysis than others. Hydrolysis occurs when water is added to a covalent peptide bond, leading to the release of individual amino acids [
52]. The complete hydrolysis of proteins to amino acids can occur under high temperatures and acidic conditions [
53]. In a neutral pH environment, hydrolysis occurs more slowly and may lead to the formation of peptides and some free amino acids depending on the intensity of the heat treatment. Therefore, the amino acids released from denatured proteins may be either free amino acids or small peptides. It is important to note that the denaturation of proteins can occur without hydrolysis, as the disruption of a protein’s three-dimensional structure does not necessarily break its covalent bonds [
52].
Our results are consistent with those obtained in previous research that showed higher amino acid concentrations in dried algae treated at high temperatures, including serine, glycine, histidine, alanine, proline, and L-tyrosine [
54]. The amount of L-tyrosine in HAO-DBRH was slightly lower than that found in the initial report by AlFadhly, N.K. et al. [
42], who reported 0.3 g of L-tyrosine/100 g dried biomass. However, the L-tyrosine obtained in this study was greater than some previous findings claiming that
Arthrospira sp. contained between 0.186 and 0.160 g of L-tyrosine per 100 g of dried biomass [
52,
53]. L-tyrosine content can differ between different algal species and depend on various environmental factors, including temperature, light, nutrients, extraction techniques, and processing. The findings of this study suggest that the hot-air-oven-drying method can be employed to increase the L-tyrosine content in
Arthrospira sp. residues. It was concluded that HAO-DBRH would be the preferred sample for further experiments due to its higher L-tyrosine content.
To optimize the production of melanin using L-tyrosine from the hydrolysate of
A. platensis residues, this study employed the use of RSM as a statistical experimental design technique. RSM is an effective tool for simultaneously optimizing medium components, such as yeast extract, soluble starch, HAO-DBRH, and CuSO
4, and evaluating their interactions. The application of RSM can provide mathematical models that aid in understanding the interplay between different levels of parameters and how to achieve the optimal level of each parameter to achieve a specific goal [
54]. This approach to optimization offers several benefits, such as reducing manufacturing times, saving operation costs, and improving output quality. This study successfully used RSM as a strategy for improving an melanin production medium to obtain the highest yield of melanin from
S. antibioticus NRRL B-1701.
The results obtained from this study, as presented in
Table 4, demonstrate a highly significant (
p < 0.01) quadratic effect of yeast extract and HAO-DBRH on melanin production, while the other factors were not significant. The effect of yeast extract and HAO-DBRH on melanin yield in the optimum medium when the concentrations of soluble starch and CuSO
4 were maintained at 3.0 and 0.0136 g/L, respectively, are illustrated in
Figure 3B. The results show that when the concentration of HAO-DBRH was constant, melanin production was increased through the incorporation of a 4.85 g/L concentration of yeast extract but remained unchanged thereafter. This finding is supported by previous studies that suggested the use of yeast extract as a nitrogen source in a medium to improve pigment synthesis in
Streptomyces chibaensis [
55]. Additionally, the use of yeast extract as a source of nitrogen for marine
Streptomyces strains that produce melanin has been explained in another study [
56].
Moreover, when the concentration of yeast extract remained constant, an increase in HAO-DBRH concentration increased melanin yield, but gradually decreased thereafter. The maximum value of melanin yield was attained when the concentration of HAO-DBRH was around 18.67 g/L, indicating that excess HAO-DBRH might reduce melanin yield. This result could be attributed to the fact that some amino acids (such as L-tyrosine) released during the hydrolysate residue process [
21] can encourage the formation of melanin and serve as a substrate in the melanin synthesis pathway [
57,
58]. This finding is in agreement with that found by Zou and Hou [
36], who demonstrated that L-tyrosine can enhance melanin production but has the opposite effect when applied in excess due to a decrease in the solubility of L-tyrosine at higher concentrations, which leads to the accumulation of the substrate in the reactor by precipitation. This accumulation can cause disruptions in the normal functioning of cells as the degradation of L-tyrosine releases ammonia [
59]. Hydrolysate is a complex mixture of oligopeptides, peptides, and free amino acids [
27]. However, the presence of inhibitory compounds in hydrolysates that may negatively affect microbial melanin production should be taken into consideration. Therefore, further studies are necessary to identify these compounds and evaluate their impacts on melanin production in order to obtain a more comprehensive understanding of the effects of hydrolysates in this process.
Therefore, the results of this study provide a better understanding of the effect of yeast extract and HAO-DBRH on melanin production, which can inform the optimization of medium components and contribute to the development of more efficient and cost-effective melanin production processes.
As a result of RSM optimization, the maximum level of melanin production was the highest in OM3 medium, which contained yeast extract, soluble starch, CuSO4, and HAO-DBRH concentrations of 5.16, 4.92, 0.0191, and 17.11 g/L, respectively. The maximum melanin production level predicted by the quadratic model was 0.164 g/L, which was verified in a validation experiment. The experimentally observed maximum melanin production level was 0.244 g/L, which could be attributed to the continuous monitoring of the absorbances at four-hour intervals, whereby the highest absorbance value was detected at 36 h.
Time is a crucial aspect in the fermentation process as a pigment can be degraded by light [
15]; therefore, melanin functions as a photoprotective pigment or can be excreted by microbes from the fermentation medium [
17]. This result differs from the findings of previous investigations [
15,
38,
60], which demonstrated that the highest melanin yield was detected after seven days. However, when comparing the degree of melanin production at 36 h, the optimized medium produced more melanin than the unoptimized medium according to Guo et al. [
37], with OM3 being the most effective, yielding around four times more melanin than the control.
The cost analysis of the medium used in the experiment was carried out based on the price of the ingredients and is presented in
Table 6. The cost of the optimized medium that produced the highest amount of melanin was USD 0.52 per gram of melanin. This is significantly less expensive compared to the previously used medium [
37], which cost USD 10.20 per gram of melanin. The use of HAO-DBRH as an ingredient in the medium was found to be a cost-effective alternative to chemical L-tyrosine and resulted in an increase in melanin production by
S. antibioticus NRRL B-107 and a reduction in the overall cost of the medium.
Studies have highlighted the significance of using non-animal protein sources in the pharmaceutical industry and the usage of protein hydrolysates from
A. platensis as a safe and effective alternative to peptone in microbial cultures. This aligns with the United Nations’ sustainable development goals (SDGs), which concern the use of natural and renewable resources to develop a bioeconomy [
61], emphasizing the importance of natural resources in achieving a sustainable future. Thus, the use of low-cost and sustainable ingredients such as HAO-DBRH in a medium is an essential step toward a more sustainable and eco-friendly approach to melanin production.
Our current research is focused on optimizing the batch system’s performance. Nevertheless, we recognize the potential benefits of using a fed-batch system, which can reduce the accumulation of byproducts and improve overall efficiency. Therefore, we plan to investigate the feasibility of this optimization approach in a fed-batch system in future research. Our goal is to contribute to sustainable development by promoting efficient and sustainable bioprocessing methods through our research.