1. Introduction
Melanomas and non-melanocytic skin cancer, primarily basal and squamous cell carcinoma, are the main types of malignant skin cancer in humans. Early detection and excision remain the most important prognostic factors for the treatment of all types of skin cancer, especially melanoma [
1]. Detection continues to be challenging and melanoma-related mortality is still high. In 2020, an estimated 65,168 people were diagnosed with melanoma [
2] and 179,219 with non-melanoma skin cancer [
3] in Western Europe. There has been a significant interest in new technologies aimed at augmenting the detection rate achieved with the clinical diagnosis of skin cancer [
4]. In the clinical risk assessment of patients with suspicious skin lesions, electrical impedance spectroscopy (EIS) proved to be a safe and accurate technical means of support, making additional information available to the physician responsible for deciding whether biopsy or excision of the lesion was indicated. EIS proved to increase detection efficacy in melanoma diagnostics [
5]. Furthermore, a good discriminative power to distinguish NMSC from benign skin tissue was shown [
6,
7]. This retrospective study was conducted to investigate the accuracy of a three-step approach involving visual examination, dermoscopy, and EIS integrated into the cancer diagnosis carried out by a dermatological practice as part of its everyday clinical routine. Validation of the Neviscore (NS), the output of the EIS device, was performed by comparing the results of the NS with histopathological findings. The sensitivity and accuracy of the technique in detecting malignancies was assessed on the basis of this comparison. The positive and practical consequence for the patients is that there may be a large reduction in the number of excisions in the future.
2. Materials and Methods
The study involved the retrospective examination of 909 lesions detected in 481 patients from a dermatological practice, who had been diagnosed from 2015 to 2017 with the help of EIS. The ethical committee of the Bavarian Medical Association reviewed the study and exempted it from ethical approval.
This study included only lesions presented in the course of the cancer consultation of the respective practice. To evaluate the nature of a lesion, a thorough visual examination was performed, followed by dermoscopy in accordance with the current dermatological standards. Lesions were evaluated using the ABCD score as described by Stolz [
8]. If the benign nature of a lesion was uncertain, EIS was used as an additional diagnostic tool.
Normal healthy skin tissue, in contrast to atypical diseased tissue, has a different cell size, shape, orientation, compactness, and structure of the cell membranes. These changes affect the cell’s ability to conduct and store electricity, a measurable property called electrical impedance. Thus, if a nevus is exposed to electrical signals during examination with Nevisense, changes can be detected using EIS and thus, melanoma and its precursors can be detected or ruled out. The technique is based on a harmless electrical signal emitted by the device which can detect and analyze these changes, allowing microinvasive bioimpedance measurements. EIS is a painless and non-invasive technology that measures the resistance in the tissue in the upper layers of the skin. Per measurement over 225 measurement points are taken and changes can be detected that indicate abnormalities in cell structure, orientation, size, molecular composition, and cell wall integrity. Incoming data are processed and classified using a complex algorithm. The EIS classifier provides an EIS score, the so-called Neviscore, on a scale of 0–10, that reflects the degree of atypia identified by the method. If the score is between 0 and 3 (negative NS), the lesion can be considered non-malignant and the patient may not have to have it removed. A score of 4 to 10 rates as positive NS, so that excision of the lesion is advisable. The examination takes only a few minutes and can be easily integrated into the normal preventive examination. During the examination, a stamp-shaped electrode is pressed twice onto the skin for each birthmark. The result in the form of the EIS score is available immediately. This makes it possible to make a decision based on reliable facts.
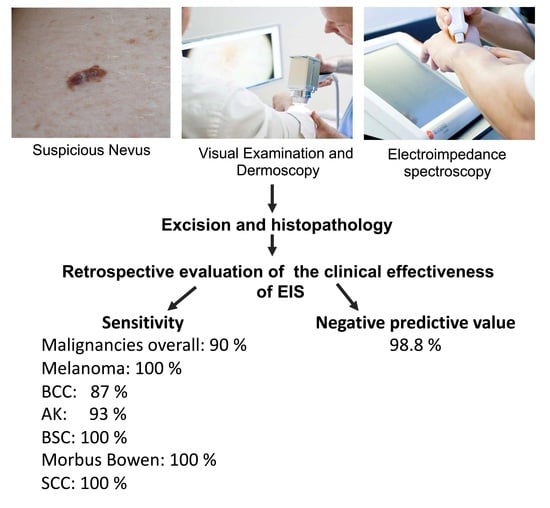
In this study, 909 lesions were suspicious for malignancy, and in addition to visual examination and dermoscopy, examined with EIS. The EIS was used as an objective, unbiased, and automated source of supportive information, and the score was taken into consideration when deciding on how to further manage the lesion. Nonetheless, the final decision about whether to operate was made on the basis of the doctor’s clinical expertise. All excised samples were sent for histopathological evaluation. In a retrospective approach, the NS of all excised lesions was compared to the definite histopathological classification to cross-check the accuracy of the assigned NS.
The next step involved taking (i) the number needed to excise (NNE), (ii) the sensitivity, and (iii) the negative predictive value (NPV) to assess the efficiency of diagnosing melanoma using the practical approach described previously [
6]. The NNE is a metric providing information about the number of biopsies performed by the dermatological practice for every malignancy diagnosed. It was calculated by dividing the total number of lesions removed by the number of confirmed malignancies (including melanoma, basal cell carcinoma (BCC), squamous cell carcinoma (SCC), morbus Bowen, and basosquamous carcinoma). By comparing the NNE both with and without the EIS result, the increase in diagnostic accuracy through using EIS could be determined.
The sensitivity shows the proportion of malignant lesions correctly identified by receiving a positive Neviscore in the clinical setting. Sensitivity was calculated by dividing the total number of malignancies with a positive Nevisense score by the total number of malignancies (
Supplementary Figure S1).
The NPV gives the ratio of true negative results compared to all negative results. Thus, the NPV was calculated as all benign lesions with a negative score (NS 0 to 3) divided by all lesions with a negative score. In this setting, the NPV shows the ratio of negatively scored lesions that were excised but were not malignant (
Supplementary Figure S1).
Lesions included in this study had to meet particular criteria to be considered suitable for evaluation with EIS. Hence, the exclusion criteria for the use of EIS based on the manufacturer’s specifications for the device were in accordance with previous studies [
6]. Included were all patients regardless of sex and age who presented to the dermatologist in the course of nevus screening or due to another reason for examination, whose nevi were classified as potentially malignant due to previous visual inspection, anamnesis, and dermoscopy. Suspicious lesions were additionally examined with Nevisense. In accordance with the manufacturer’s guidelines, some lesions were excluded from EIS evaluation. The exclusion criteria for the lesions studied were: (i) metastases of a pre-existing malignancy, (ii) a diameter of less than 2 mm or more than 20 mm, (iii) lesions on obviously non-intact skin, for example, in the form of eczema, scars, sunburn, previous injuries, trauma, etc., (iv) lesions on extremely hairy skin such as the scalp or beard, (v) lesions on the genital area or other mucosal location, (vi) lesions on tattoos, or in the presence of other foreign bodies or unphysiological interfering factors, and (vii) pedunculated lesions.
Limitations of the Study
Not all lesions that were considered suspicious for malignancy and that had a positive Neviscore were excised. This was due to the fact that some patients declined having an intervention. Most lesions that scored negative after EIS examination were not excised in order to spare the patient the intervention. Nonetheless, patients with skin abnormalities were monitored closely and asked to return for repeated examination in short intervals. However, because of the initial study design, the result of follow-up examinations was not reported and not considered when analyzing the data. Thus, no objective statement can be made whether malignant changes were present in lesions with a negative Neviscore.
4. Discussion
This study was based on the everyday procedures of a private dermatology practice. In total, 45% of suspicious lesions were removed after visual and EIS testing; 16% of these were found through histopathological examination to be malignant. The EIS testing process proved to be 100% accurate in identifying melanomas, although these were extremely rare. This retrospective study showed how EIS was integrated into the everyday clinical routine and how rare melanomas were reliably detected through the additional use of EIS. Patients could be spared a high percentage of the excisions performed in the course of skin cancer prevention.
The EIS technology and its use in cancer detection has been investigated in several previous studies. In a prospective blinded clinical trial on the efficacy and safety of EIS [
9] that involved 1943 eligible equivocal lesions that would have been excised on the basis of visual evaluation, EIS achieved a sensitivity for melanoma of 96.6% and for non-melanoma skin cancer of 100%, and a specificity of 34.1%. In another study, EIS was used in a protocol for routine sequential digital dermoscopy imaging for a total of 160 lesions [
10]. The addition of EIS to the protocol reduced the need for sequential monitoring by 47% and also identified 83% of the melanomas three months earlier than the standard sequential monitoring protocol. In a reader study, 164 clinicians were shown clinical images of lesions, and made a total of 7380 clinical decisions with and without the EIS output [
11]. When the EIS results were included, the mean sensitivity improved from 80.7% to 95.2% and the mean specificity from 50.4% to 58.6%. The non-melanoma skin cancer assessment was also highly accurate [
9,
10,
12]. In accordance with previous findings, the results of this study confirm that the EIS system is highly reliable with regard to the detection of malign skin aberrations, especially melanoma.
The NNE is a key indicator of the clinical utility of EIS for the dermatologist and of the benefits for the patients. In this investigation, the NNE necessary for identifying one malignant lesion dropped significantly, while the efficiency in detecting malignant lesions increased. If the malignant potential of a suspicious lesion was in doubt, the EIS system predicted with a degree of high sensitivity whether a lesion needed to be excised. For the patients, this means that fewer lesions that are benign or mildly to moderately dysplastic are excised in the first place. Fewer surgical interventions are necessary, reducing the burden for the patients.
A more current guideline for the integration of EIS in the screening process for skin cancer was described in 2018 [
13], and this process is in addition supported by Onkoderm—a German dermato-oncology association. According to the guideline, lesions scoring an NS of 0 to 3 can be considered as benign. Lesions with an NS of 4 to 6 are not in need of immediate excision but should be followed-up. The guideline recommends that lesions with an NS of 7 to 10 should be excised immediately. These recommendations can of course be overthrown by the expert opinion of the dermatologist. Our results support this approach and indicate the usefulness of integrating EIS into the screening process for skin cancer as described in the guideline.
This study provided insight into the outcome of using EIS in a single private practice. It is important to emphasize that the cohort of patients and lesions included in this study was seen during normal medical consultation over a defined time period. The prevalence of malignant lesions was lower than in the previous controlled clinical studies but considering the patient population in the clinic and the usage of the device on lesions with a lower suspicion of malignancy or dysplasia as well, this was to be expected. Since this was a retrospective analysis, the definite diagnosis of lesions that were left untreated was unknown and no reliable value for false negative results could be calculated. We were also confronted with a further limitation: several patients refused to undergo invasive treatment and have positively scored lesions excised. Another limitation of the study is the lack of follow-up. There was no evaluation available on the non-biopsy patients whether malignant skin cancer was detected at a later stage. This impeded an all-encompassing statistical evaluation with respect to, for example, the correlation between NS and histopathological diagnosis. Nonetheless, we found it important to include the data in its entirety to portray the everyday routine of a clinical practice. The EIS device nevertheless provided useful information to support a doctor’s recommendation regarding the surgical removal of a lesion [
14,
15]. The doctor’s decision is ultimately based on his or her experience. The unbiased and objective additional information of the NS can be helpful for deciding which lesions to excise and can contribute to reducing unnecessary excisions. This is particularly beneficial for people with several suspicious lesions and poor wound healing. As extensive studies have confirmed, the automated and highly standardized determination of the NS in addition to the subjective visual examination is a quick and reliable means of indicating the probability of malignancy. This makes the additional examination using EIS a suitable instrument for cancer screening and for monitoring the progress of suspicious lesions.