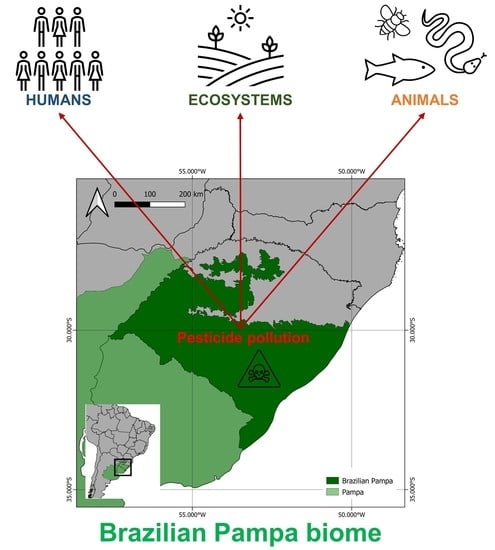
Pesticide Pollution in the Brazilian Pampa: Detrimental Impacts on Ecosystems and Human Health in a Neglected Biome
Abstract
:1. Environmental Health and the Pampa Biome
2. Pesticide Pollution in the Pampa Biome
2.1. Current Scenario
2.1.1. Pesticides in Water
2.1.2. Pesticides in Soil
2.1.3. A Neglected and Evolving Problem
2.2. Impacts on Animals and Ecosystems
2.3. Consequences of Pesticides on Human Health
3. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prüss-Ustün, A.; Wolf, J.; Corvalán, C.; Bos, R.; Neira, M. Preventing Disease through Healthy Environments: A Global Assessment of the Burden of Disease from Environmental Risks; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Ellwanger, J.H.; Byrne, L.B.; Chies, J.A.B. Examining the paradox of urban disease ecology by linking the perspectives of Urban One Health and Ecology with Cities. Urban Ecosyst. 2022, 25, 1735–1744. [Google Scholar] [CrossRef]
- Essack, S.Y. Environment: The neglected component of the One Health triad. Lancet Planet. Health 2018, 2, e238–e239. [Google Scholar] [CrossRef]
- Ellwanger, J.H.; Kulmann-Leal, B.; Kaminski, V.L.; Valverde-Villegas, J.M.; Veiga, A.B.G.; Spilki, F.R.; Fearnside, P.M.; Caesar, L.; Giatti, L.L.; Wallau, G.L.; et al. Beyond diversity loss and climate change: Impacts of Amazon deforestation on infectious diseases and public health. An. Acad. Bras. Cienc. 2020, 92, e20191375. [Google Scholar] [CrossRef]
- Fuller, R.; Landrigan, P.J.; Balakrishnan, K.; Bathan, G.; Bose-O’Reilly, S.; Brauer, M.; Caravanos, J.; Chiles, T.; Cohen, A.; Corra, L.; et al. Pollution and health: A progress update. Lancet Planet. Health 2022, 6, e535–e547. [Google Scholar] [CrossRef]
- Fuller, R.; Sandilya, K.; Hanrahan, D. Pollution and Health Metrics. Global, Regional, and Country Analysis December 2019; Global Alliance on Health and Pollution (GAHP): New York, NY, USA, 2019. [Google Scholar]
- Clarke, R.M.; Cummins, E. Evaluation of “classic” and emerging contaminants resulting from the application of biosolids to agricultural lands: A review. Hum. Ecol. Risk Assess. 2015, 21, 492–513. [Google Scholar] [CrossRef]
- Bertoldi, C.; Lara, L.Z.; Mizushima, F.A.L.; Martins, F.C.G.; Battisti, M.A.; Hinrichs, R.; Fernandes, A.N. First evidence of microplastic contamination in the freshwater of Lake Guaíba, Porto Alegre, Brazil. Sci. Total Environ. 2021, 759, 143503. [Google Scholar] [CrossRef]
- Tang, F.H.M.; Lenzen, M.; McBratney, A.; Maggi, F. Risk of pesticide pollution at the global scale. Nat. Geosci. 2021, 14, 206–210. [Google Scholar] [CrossRef]
- Wilson, C.; Tisdell, C. Why farmers continue to use pesticides despite environmental, health and sustainability costs. Ecol. Econ. 2001, 39, 449–462. [Google Scholar] [CrossRef]
- Atreya, K.; Sitaula, B.K.; Johnsen, F.H.; Bajracharya, R.M. Continuing issues in the limitations of pesticide use in developing countries. J. Agric. Environ. Ethics 2011, 24, 49–62. [Google Scholar] [CrossRef]
- Tudi, M.; Ruan, H.D.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture development, pesticide application and its impact on the environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef]
- Roesch, L.F.W.; Vieira, F.C.B.; Pereira, V.A.; Schünemann, A.L.; Teixeira, I.F.; Senna, A.J.T.; Stefenon, V.M. The Brazilian Pampa: A fragile biome. Diversity 2009, 1, 182–198. [Google Scholar] [CrossRef]
- Overbeck, G.E.; Vélez-Martin, E.; Menezes, L.S.; Anand, M.; Baeza, S.; Carlucci, M.B.; Dechoum, M.S.; Durigan, G.; Fidelis, A.; Guido, A.; et al. Placing Brazil’s grasslands and savannas on the map of science and conservation. Perspect. Plant Ecol. Evol. Syst. 2022, 56, 125687. [Google Scholar] [CrossRef]
- Müller, S.C.; Overbeck, G.E.; Blanco, C.C.; de Oliveira, J.M.; Pillar, V.D. South Brazilian Forest-Grassland Ecotones: Dynamics Affected by Climate, Disturbance, and Woody Species Traits. In Ecotones Between Forest and Grassland; Myster, R.D., Ed.; Springer: New York, NY, USA, 2012; pp. 167–187. [Google Scholar] [CrossRef]
- Copatti, C.E.; do Amaral, A.D.; de Moura, C.F.A. Aves em ecótono Mata Atlântica-Pampa no Sul do Brasil. Ciência Nat. 2013, 35, 30–40. [Google Scholar] [CrossRef]
- Viera, L.F.S. A Valoração da Beleza Cênica da paisagem do Bioma Pampa do Rio Grande do Sul: Proposição Conceitual e Metodológica. Ph.D. Thesis, Instituto de Geociências, Programa de Pós-Graduação em Geografia, Universidade Federal do Rio Grande do Sul (UFRGS), Porto Alegre, Brazil, 2014. [Google Scholar]
- Porto, A.B.; Rolim, R.G.; da Silveira, F.F.; Overbeck, G.E.; Salatino, A. Consciência Campestre: Um chamado para o (re)conhecimento aos campos. Bio Diverso 2021, 1, 164–188. [Google Scholar]
- Overbeck, G.E.; Vélez-Martin, E.; Scarano, F.R.; Lewinsohn, T.M.; Fonseca, C.R.; Meyer, S.T.; Müller, S.C.; Ceotto, P.; Dadalt, L.; Durigan, G.; et al. Conservation in Brazil needs to include non-forest ecosystems. Divers. Distrib. 2015, 21, 1455–1460. [Google Scholar] [CrossRef]
- Mazurana, J.; Dias, J.E.; Laureano, L.C. Povos e Comunidades Tradicionais do Pampa; Fundação Luterana de Diaconia: Porto Alegre, Brazil, 2016. [Google Scholar]
- Andrade, B.O.; Dröse, W.; de Aguiar, C.A.; Aires, E.T.; Alvares, D.J.; Barbieri, R.L.; de Carvalho, C.J.B.; Bartz, M.; Becker, F.G.; Benck, G.A.; et al. 12,500+ and counting: Biodiversity of the Brazilian Pampa. Front. Biogeogr. 2023, 15, e59288. [Google Scholar] [CrossRef]
- Krob, A.D.; Overbeck, G.E.; Mähler, J.J.F., Jr.; Urruth, L.M.; Malabarba, L.R.; Chomenko, L.; Azevedo, M.A. Contribution of southern Brazil to the climate and biodiversity conservation agenda. Bio Diverso 2021, 1, 132–144. [Google Scholar]
- Stumpf, E.T.; Romano, C.M.; Barbieri, R.L.; Heiden, G.; Fischer, S.Z.; Corrêa, L.B. Características ornamentais de plantas do Bioma Pampa. Ornam. Hortic. 2009, 15, 49–62. [Google Scholar] [CrossRef]
- Carrion, A.A.; Brack, P. Eudicotiledôneas ornamentais dos campos do bioma Pampa no Rio Grande do Sul. Ornam. Hortic. 2012, 18, 23–37. [Google Scholar] [CrossRef]
- Severo, S.A.; da Silva, L.F.; Trevisan, A.C.D. Plantas nativas da sociobidiversidade do bioma Pampa. Salão Integr. Ensino Pesqui. Extensão Uergs 2021, 1, 10. [Google Scholar]
- De Souza, B.R. Cosmética Para o SocioBioCotidiano: Uma Análise da Emergência da Cadeia de Cosméticos Ecológicos a Partir da Flora Nativa dos Biomas Pampa e Mata Atlântica Sul, Brasil. Universidade Federal do Rio Grande do Sul—UFRGS. 2022. Available online: https://lume.ufrgs.br/bitstream/handle/10183/240086/001141934.pdf?sequence=1&isAllowed=y (accessed on 25 February 2023).
- MapBiomas—Mapeamento Anual de Cobertura e Uso da Terra no Pampa—Coleção 7. Destaques do Mapeamento Anual da Cobertura e uso da Terra no Brasil de 1985 a 2021: Pampa. 2022. Available online: https://mapbiomas-br-site.s3.amazonaws.com/MapBiomas_PAMPA_2022_11.10__1_.pdf (accessed on 22 February 2023).
- Echer, R.; da Cruz, J.A.W.; Estrela, C.C.; Moreira, M.; Gravato, F. Usos da terra e ameaças para a conservação da biodiversidade no bioma Pampa, Rio Grande do Sul. Rev. Thema 2015, 12, 4–13. [Google Scholar] [CrossRef]
- Lima, J.A.M.C.; Labanowski, J.; Bastos, M.C.; Zanella, R.; Prestes, O.D.; de Vargas, J.P.R.; Mondamert, L.; Granado, E.; Tiecher, T.; Zafar, M.; et al. “Modern agriculture” transfers many pesticides to watercourses: A case study of a representative rural catchment of southern Brazil. Environ. Sci. Pollut. Res. Int. 2020, 27, 10581–10598. [Google Scholar] [CrossRef] [PubMed]
- Ellwanger, J.H.; Ziliotto, M.; Chies, J.A.B. Protect Brazil’s overlooked Pampa biome. Science 2022, 377, 720. [Google Scholar] [CrossRef] [PubMed]
- Governo do Estado do Rio Grande do Sul, Secretaria de Planejamento, Governança e Gestão, Atlas Econoômico do Rio Grande do Sul, Índice de Desenvolvimento Humano—IDH e IDHM. 2022. Available online: https://atlassocioeconomico.rs.gov.br/indice-de-desenvolvimento-humano-idh-e-idhm (accessed on 11 April 2023).
- Overbeck, G.E.; Müller, S.C.; Fidelis, A.; Pfadenhauer, J.; Pillar, V.D.; Blanco, C.C.; Boldrini, I.I.; Both, R.; Forneck, E.D. Brazil’s neglected biome: The South Brazilian Campos. Perspect. Plant Ecol. Evol. Syst. 2007, 9, 101–116. [Google Scholar] [CrossRef]
- Albuquerque, A.F.; Ribeiro, J.S.; Kummrow, F.; Nogueira, A.J.A.; Montagner, C.C.; Umbuzeiro, G.A. Pesticides in Brazilian freshwaters: A critical review. Environ. Sci. Process Impacts 2016, 18, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.L.F.; Volcão, L.M.; Ramires, P.F.; Moura, R.R.; Da Silva Júnior, F.M.R. Distribution of pesticides in agricultural and urban soils of Brazil: A critical review. Environ. Sci. Process Impacts 2020, 22, 256–270. [Google Scholar] [CrossRef]
- Pignati, W.A.; Lima, F.A.N.S.; Lara, S.S.; Correa, M.L.M.; Barbosa, J.R.; Leão, L.H.C.; Pignatti, M.G. Spatial distribution of pesticide use in Brazil: A strategy for Health Surveillance. Cien. Saude Colet. 2017, 22, 3281–3293. [Google Scholar] [CrossRef]
- Burity, V.T.A.; Melgarejo, L.; Gonzáles, J.C.M.; Prates, L.A.; Rocha, N.C. Pesticides in Latin America: Violations against the Right to Adequate Food and Nutrition, 1st ed.; FIAN Brasil: Brasília, Brazil, 2020. [Google Scholar]
- Kuhn, E.C. Avaliação Ecotoxicológica do rio Uruguay e Efluentes pré e pós Aplicação de Pesticidas Utilizando Caenorhabditis elegans Como Biomonitor. 2018. Universidade Federal do Pampa–UNIPAMPA. Available online: https://dspace.unipampa.edu.br/bitstream/riu/4970/1/EUG%c3%8aNIA%20CARLA%20KUHN.pdf (accessed on 26 February 2023).
- Kemmerich, M.; Bernardi, G.; Adaime, M.B.; Zanella, R.; Prestes, O.D. A simple and efficient method for imidazolinone herbicides determination in soil by ultra-high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2015, 1412, 82–89. [Google Scholar] [CrossRef]
- Coelho, F.E.A.; Lopes, L.C.; Cavalcante, R.M.S.; Corrêa, G.C.; Leduc, A.O.H.C. Brazil unwisely gives pesticides a free pass. Science 2019, 365, 552–553. [Google Scholar] [CrossRef]
- Carneiro, L.; Faria, L.; Miiller, N.; Cavalcante, A.; Murata, A.; Vitule, J.R.S. Brazilian pesticides law could poison the world. Science 2022, 376, 362. [Google Scholar] [CrossRef]
- Schaffner, R. Facilitação do Registro de Agrotóxicos Amplia Debate Sobre Impactos dos Produtos. 2022. Available online: https://gauchazh.clicrbs.com.br/politica/noticia/2022/02/facilitacao-do-registro-de-agrotoxicos-amplia-debate-sobre-impactos-dos-produtos-ckzhis00m001b0188o4z7ry7m.html (accessed on 24 February 2023).
- Viglizzo, E.F.; Franke, F.C.; Carreño, L.V.; Jobbágy, E.G.; Pereyra, H.; Clatt, J.; Pincén, D.; Ricard, M.F. Ecological and environmental footprint of 50 years of agricultural expansion in Argentina. Glob. Chang. Biol. 2011, 17, 959–973. [Google Scholar] [CrossRef]
- Iturburu, F.G.; Calderon, G.; Amé, M.V.; Menone, M.L. Ecological Risk Assessment (ERA) of pesticides from freshwater ecosystems in the Pampas region of Argentina: Legacy and current use chemicals contribution. Sci. Total Environ. 2019, 691, 476–482. [Google Scholar] [CrossRef]
- Pérez, D.J.; Iturburu, F.G.; Calderon, G.; Oyesqui, L.A.E.; De Gerónimo, E.; Aparicio, V.C. Ecological risk assessment of current-use pesticides and biocides in soils, sediments and surface water of a mixed land-use basin of the Pampas region, Argentina. Chemosphere 2021, 263, 128061. [Google Scholar] [CrossRef]
- Harriet, J.; Campá, J.P.; Grajales, M.; Lhéritier, C.; Pajuelo, A.G.; Mendoza-Spina, Y.; Carrasco-Letelier, L. Agricultural pesticides and veterinary substances in Uruguayan beeswax. Chemosphere 2017, 177, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Bolaña, C.; Pérez-Parada, A.; Tesitore, G.; Goyenola, G.; Kröger, A.; Pacheco, M.; Gérez, N.; Berton, A.; Zinola, G.; Gil, G.; et al. Multicompartmental monitoring of legacy and currently used pesticides in a subtropical lake used as a drinking water source (Laguna del Cisne, Uruguay). Sci. Total Environ. 2023, 874, 162310. [Google Scholar] [CrossRef]
- Delcour, I.; Spanoghe, P.; Uyttendaele, M. Literature review: Impact of climate change on pesticide use. Food Res. Int. 2015, 68, 7–15. [Google Scholar] [CrossRef]
- Galon, L.; Bragagnolo, L.; Korf, E.P.; Dos Santos, J.B.; Barroso, G.M.; Ribeiro, V.H.V. Mobility and environmental monitoring of pesticides in the atmosphere—A review. Environ. Sci. Pollut. Res. Int. 2021, 28, 32236–32255. [Google Scholar] [CrossRef]
- Köhler, H.R.; Triebskorn, R. Wildlife ecotoxicology of pesticides: Can we track effects to the population level and beyond? Science 2013, 341, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Kiesecker, J.M. Global stressors and the global decline of amphibians: Tipping the stress immunocompetency axis. Ecol. Res. 2011, 26, 897–908. [Google Scholar] [CrossRef]
- Rohr, J.R.; Schotthoefer, A.M.; Raffel, T.R.; Carrick, H.J.; Halstead, N.; Hoverman, J.T.; Johnson, C.M.; Johnson, L.B.; Lieske, C.; Piwoni, M.D.; et al. Agrochemicals increase trematode infections in a declining amphibian species. Nature 2008, 455, 1235–1239. [Google Scholar] [CrossRef] [PubMed]
- Lopes, C.V.A.; Albuquerque, G.S.C. Agrotóxicos e seus impactos na saúde humana e ambiental: Uma revisão sistemática. Saúde Debate 2018, 42, 518–534. [Google Scholar] [CrossRef]
- Gill, R.J.; Ramos-Rodriguez, O.; Raine, N.E. Combined pesticide exposure severely affects individual- and colony-level traits in bees. Nature 2012, 491, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Kakumanu, M.L.; Reeves, A.M.; Anderson, T.D.; Rodrigues, R.R.; Williams, M.A. Honey bee gut microbiome is altered by in-hive pesticide exposures. Front. Microbiol. 2016, 7, 1255. [Google Scholar] [CrossRef] [PubMed]
- Costa-Silva, D.G.; Nunes, M.E.M.; Wallau, G.L.; Martins, I.K.; Zemolin, A.P.P.; Cruz, L.C.; Rodrigues, N.R.; Lopes, A.R.; Posser, T.; Franco, J.L. Oxidative stress markers in fish (Astyanax sp. and Danio rerio) exposed to urban and agricultural effluents in the Brazilian Pampa biome. Environ. Sci. Pollut. Res. Int. 2015, 22, 15526–15535. [Google Scholar] [CrossRef]
- Nunes, M.; da Silva, F.W.; Costa-Silva, D.; Wallau, G.L.; Posser, T.; Franco, J.L. Assessment of water pollution signs in the Brazilian Pampa biome using stress biomarkers in fish (Astyanax sp.). J. Ecosyst. 2015, 2015, 415293. [Google Scholar] [CrossRef]
- Gonçalves, C.; Marins, A.T.; do Amaral, A.M.B.; Nunes, M.E.M.; Müller, T.E.; Severo, E.; Feijó, A.; Rodrigues, C.C.R.; Zanella, R.; Prestes, O.D.; et al. Ecological impacts of pesticides on Astyanax jacuhiensis (Characiformes: Characidae) from the Uruguay river, Brazil. Ecotoxicol. Environ. Saf. 2020, 205, 111314. [Google Scholar] [CrossRef] [PubMed]
- Severo, E.S.; Marins, A.T.; Cerezer, C.; Costa, D.; Nunes, M.; Prestes, O.D.; Zanella, R.; Loro, V.L. Ecological risk of pesticide contamination in a Brazilian river located near a rural area: A study of biomarkers using zebrafish embryos. Ecotoxicol. Environ. Saf. 2020, 190, 110071. [Google Scholar] [CrossRef] [PubMed]
- Severo, E.; Marins, A.; de Menezes, C.; Nunes, M.; Murussi, C.; da Costa-Silva, D.G.; Storck, T.R.; Prestes, O.D.; Adaime, M.B.; Loro, V.L.; et al. Biomarkers’ responses of Rhamdia quelen exposed in situ on a Brazilian river located in agricultural areas. Water Air Soil Pollut. 2023, 234, 144. [Google Scholar] [CrossRef]
- Santos, T.G.; Melo, R.; Costa-Silva, D.G.; Nunes, M.E.M.; Rodrigues, N.R.; Franco, J.L. Assessment of water pollution in the Brazilian Pampa biome by means of stress biomarkers in tadpoles of the leaf frog Phyllomedusa iheringii (Anura: Hylidae). PeerJ 2015, 3, e1016. [Google Scholar] [CrossRef]
- Pires, M.M.; Sahlén, G.; Périco, E. Agricultural land use affects the heterogeneity of Odonata communities in the Brazilian Pampa. J. Insect Conserv. 2022, 26, 503–514. [Google Scholar] [CrossRef]
- Rodrigues, C.S.; Ferasso, D.C.; Prestes, O.D.; Zanella, R.; Grando, R.C.; Treichel, H.; Coelho, G.C.; Mossi, A.J. Quality of Meliponinae honey: Pesticides residues, pollen identity, and microbiological profiles. Environ. Qual. Manag. 2018, 27, 39–45. [Google Scholar] [CrossRef]
- Grigori, P. Half a Billion Bees Dead as Brazil Approves Hundreds more Pesticides. 2019. Available online: https://news.mongabay.com/2019/08/half-a-billion-bees-dead-as-brazil-approves-hundreds-more-pesticides/ (accessed on 24 February 2023).
- Caesar, L. Síndrome Anual da Abelha Mandaçaia (Melipona quadrifasciata)—O Papel de Simbiontes, Sistema Imune e Ambiente. Ph.D. Thesis, Postgraduate Program in Genetics and Molecular Biology, Universidade Federal do Rio Grande do Sul—UFRGS, Porto Alegre, Brazil, 2020. Available online: https://lume.ufrgs.br/bitstream/handle/10183/212107/001116139.pdf?sequence=1&isAllowed=y (accessed on 25 February 2023).
- EFSA Scientific Committee. Guidance to develop specific protection goals options for environmental risk assessment at EFSA, in relation to biodiversity and ecosystem services. EFSA J. 2016, 14, 4499. [Google Scholar] [CrossRef]
- Nienstedt, K.M.; Brock, T.C.; van Wensem, J.; Montforts, M.; Hart, A.; Aagaard, A.; Alix, A.; Boesten, J.; Bopp, S.K.; Brown, C.; et al. Development of a framework based on an ecosystem services approach for deriving specific protection goals for environmental risk assessment of pesticides. Sci. Total Environ. 2012, 415, 31–38. [Google Scholar] [CrossRef]
- Rigotto, R.M.; Vasconcelos, D.P.; Rocha, M.M. Pesticide use in Brazil and problems for public health. Cad. Saude Publica 2014, 30, 1360–1362. [Google Scholar] [CrossRef]
- Buralli, R.J.; Souza, F.N.F. Mortality and morbidity by work-related pesticide poisoning in Brazil, 2009–2019. In ISEE Conference Abstracts; National Institute of Environmental Health Sciences: Durham, NC, USA, 2021. [Google Scholar]
- Freitas, A.B.; Garibotti, V. Characterization of notifications of exogenous pesticide poisoning in Rio Grande do Sul, Brazil, 2011–2018. Epidemiol. Serv. Saude 2020, 29, e2020061. [Google Scholar] [CrossRef]
- Pacheco, A.O.; Hackel, C. Instabilidade cromossômica induzida por agroquímicos em trabalhadores rurais na região de Passo Fundo, Rio Grande do Sul, Brasil. Cad. Saúde Pública 2002, 18, 1675–1683. [CrossRef]
- Bortoli, G.M.; Azevedo, M.B.; Silva, L.B. Cytogenetic biomonitoring of Brazilian workers exposed to pesticides: Micronucleus analysis in buccal epithelial cells of soybean growers. Mutat. Res. 2009, 675, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Remor, A.P.; Totti, C.C.; Moreira, D.A.; Dutra, G.P.; Heuser, V.D.; Boeira, J.M. Occupational exposure of farm workers to pesticides: Biochemical parameters and evaluation of genotoxicity. Environ. Int. 2009, 35, 273–278. [Google Scholar] [CrossRef]
- Benedetti, D.; Nunes, E.; Sarmento, M.; Porto, C.; dos Santos, C.E.I.; Dias, J.F.; da Silva, J. Genetic damage in soybean workers exposed to pesticides: Evaluation with the comet and buccal micronucleus cytome assays. Mutat. Res. 2013, 752, 28–33. [Google Scholar] [CrossRef]
- Hayden, K.M.; Norton, M.C.; Darcey, D.; Østbye, T.; Zandi, P.P.; Breitner, J.C.S.; Welsh-Bohmer, K.A.; Cache County Study Investigators. Occupational exposure to pesticides increases the risk of incident AD: The Cache County study. Neurology 2010, 74, 1524–1530. [Google Scholar] [CrossRef]
- Ascherio, A.; Chen, H.; Weisskopf, M.G.; O’Reilly, E.; McCullough, M.L.; Calle, E.E.; Schwarzschild, M.A.; Thun, M.J. Pesticide exposure and risk for Parkinson’s disease. Ann. Neurol. 2006, 60, 197–203. [Google Scholar] [CrossRef]
- Hancock, D.B.; Martin, E.R.; Mayhew, G.M.; Stajich, J.M.; Jewett, R.; Stacy, M.A.; Scott, B.L.; Vance, J.M.; Scott, W.K. Pesticide exposure and risk of Parkinson’s disease: A family-based case-control study. BMC Neurol. 2008, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Ellwanger, J.H.; Molz, P.; Dallemole, D.R.; Pereira dos Santos, A.; Müller, T.E.; Cappelletti, L.; Gonçalves da Silva, M.; Franke, S.I.; Prá, D.; Pêgas Henriques, J.A. Selenium reduces bradykinesia and DNA damage in a rat model of Parkinson’s disease. Nutrition 2015, 31, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Zhang, Y.; Liu, L.; Yan, H. Pesticide exposure and risk of Alzheimer’s disease: A systematic review and meta-analysis. Sci. Rep. 2016, 6, 32222. [Google Scholar] [CrossRef] [PubMed]
- Alavanja, M.C.; Ross, M.K.; Bonner, M.R. Increased cancer burden among pesticide applicators and others due to pesticide exposure. CA Cancer J. Clin. 2013, 63, 120–142. [Google Scholar] [CrossRef]
- Faria, N.M.X.; Fassa, A.G.; Meucci, R.D. Association between pesticide exposure and suicide rates in Brazil. Neurotoxicology 2014, 45, 355–362. [Google Scholar] [CrossRef]
- Franck, M.C.; Monteiro, M.G.; Limberger, R.P. Suicide mortality in Rio Grande do Sul, Brazil: A cross-sectional analysis of cases, 2017-2018. Epidemiol. Serv. Saude 2020, 29, e2019512. [Google Scholar] [CrossRef]
- Meneghel, S.N.; Victora, C.G.; Faria, N.M.X.; Carvalho, L.A.; Falk, J.W. Epidemiological aspects of suicide in Rio Grande do Sul, Brazil. Rev. Saude Publica 2004, 38, 804–810. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef]
- Rohr, J.R.; Barrett, C.B.; Civitello, D.J.; Craft, M.E.; Delius, B.; DeLeo, G.A.; Hudson, P.J.; Jouanard, N.; Nguyen, K.H.; Ostfeld, R.S.; et al. Emerging human infectious diseases and the links to global food production. Nat. Sustain. 2019, 2, 445–456. [Google Scholar] [CrossRef]
- Ellwanger, J.H.; Fearnside, P.M.; Ziliotto, M.; Valverde-Villegas, J.M.; da Veiga, A.B.G.; Vieira, G.F.; Bach, E.; Cardoso, J.C.; Müller, N.F.D.; Lopes, G.; et al. Synthesizing the connections between environmental disturbances and zoonotic spillover. An. Acad. Bras. Cienc. 2022, 94, e20211530. [Google Scholar] [CrossRef]
- Oliveira, T.E.; de Freitas, D.S.; Gianezini, M.; Ruviaro, C.F.; Zago, D.; Mércio, T.Z.; Dias, E.A.; Lampert, V.N.; Barcellos, J.O.J. Agricultural land use change in the Brazilian Pampa Biome: The reduction of natural grasslands. Land Use Policy 2017, 63, 394–400. [Google Scholar] [CrossRef]
- Ziliotto, M.; Ellwanger, J.H.; Chies, J.A.B. Geo-helmintíases no Rio Grande do Sul: Uma análise a partir da perspectiva de Saúde Única. Bio Diverso 2022, 2, 66–94. [Google Scholar]
- Pereira, E.M. Movimentos ambientalistas no Rio Grande do Sul (décadas 1970-80). Oficina Hist. 2018, 11, 21–42. [Google Scholar] [CrossRef]
- Menegassi, D. Coalizão pelo Pampa Publica Carta Aberta em Defesa do Bioma e Alerta Para Ameaças. 2022. Available online: https://oeco.org.br/noticias/coalizao-pelo-pampa-publica-carta-aberta-em-defesa-do-bioma-e-alerta-para-ameacas/ (accessed on 10 February 2023).
- Instituto Curicaca. 2020. Available online: https://www.curicaca.org.br/ (accessed on 10 February 2023).
- Rede Campos Sulinos. 2023. Available online: https://www.ufrgs.br/redecampossulinos/ (accessed on 10 February 2023).
- Comitê dos Povos e Comunidades Tradicionais do Pampa. 2023. Available online: https://comitepampa.com.br/ (accessed on 10 February 2023).
- Secretaria da Agricultura, Pecuária, Produção Sustentável e Irrigação. Agrotóxicos. 2016. Available online: https://www.agricultura.rs.gov.br/agrotoxicos-2016-12 (accessed on 20 March 2023).
- Garibotti, V. Os agrotóxicos e o direito de escolha dos cidadãos. In Boletim Epidemiológico; Centro Estadual de Vigilância em Saúde do Rio Grande do Sul: Porto Alegre, Brazil, 2012; Volume 14, pp. 1–3. [Google Scholar]
- Dapper, V.; Nussbaumer, L. Avaliação dos registros de intoxicações por agrotóxicos no Rio Grande do Sul. In Boletim Epidemiológico; Centro Estadual de Vigilância em Saúde do Rio Grande do Sul: Porto Alegre, Brazil, 2012; Volume 14, pp. 6–8. [Google Scholar]
- Martil, G.C.D.; dos Anjos, F.S. Redes agroalimentares alternativas e consumo crítico: O caso das feiras orgânicas de Porto Alegre. Política Soc. 2020, 19, 172–203. [Google Scholar] [CrossRef]
- Klein, A.D.; Klein, C.R.M.; Schultz, G. Os canais de distribuição on-line de alimentos orgânicos na região metropolitana em Porto Alegre. Grifos 2022, 31, 57. [Google Scholar] [CrossRef]
- Schneider, S.; Gazolla, M. Cadeias Curtas e Redes Agroalimentares Alternativas: Negócios e Mercados da Agricultura Familiar; Editora da UFRGS: Porto Alegre, Brazil, 2017. [Google Scholar]
- Bugge, M.M.; Hansen, T.; Klitkou, A. What Is the Bioeconomy? A Review of the Literature. Sustainability 2016, 8, 691. [Google Scholar] [CrossRef]
- Ellwanger, J.H.; Nobre, C.A.; Chies, J.A.B. Brazilian Biodiversity as a Source of Power and Sustainable Development: A Neglected Opportunity. Sustainability 2023, 15, 482. [Google Scholar] [CrossRef]
- IBGE—Instituto Brasileiro de Geografia e Estatística. Sistema de Contas Regionais: Brasil 2020. In Contas Nacionais, n. 90; IBGE: Rio de Janeiro, Brazil, 2022; pp. 1–12. [Google Scholar]
- Balestrin, A. RS, Primeiro em Inovação no Brasil. 2022. Available online: https://estado.rs.gov.br/rs-primeiro-em-inovacao-no-brasil#:~:text=Na%20produ%C3%A7%C3%A3o%20cient%C3%ADfica%2C%20o%20nosso,e%20depositam%20centenas%20de%20patentes (accessed on 10 February 2023).
- Instituto Brasileiro de Geografia e Estatística—IBGE. 2023. Available online: https://www.ibge.gov.br/ (accessed on 19 March 2023).
- Projeto MapBiomas Pampa Trinacional—Coleção 2.0 da Série Anual de Mapas de Uso e Cobertura do Solo. 2023. Available online: https://pampa.mapbiomas.org/pt-BR (accessed on 11 April 2023).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziliotto, M.; Kulmann-Leal, B.; Roitman, A.; Bogo Chies, J.A.; Ellwanger, J.H. Pesticide Pollution in the Brazilian Pampa: Detrimental Impacts on Ecosystems and Human Health in a Neglected Biome. Pollutants 2023, 3, 280-292. https://doi.org/10.3390/pollutants3020020
Ziliotto M, Kulmann-Leal B, Roitman A, Bogo Chies JA, Ellwanger JH. Pesticide Pollution in the Brazilian Pampa: Detrimental Impacts on Ecosystems and Human Health in a Neglected Biome. Pollutants. 2023; 3(2):280-292. https://doi.org/10.3390/pollutants3020020
Chicago/Turabian StyleZiliotto, Marina, Bruna Kulmann-Leal, Alice Roitman, José Artur Bogo Chies, and Joel Henrique Ellwanger. 2023. "Pesticide Pollution in the Brazilian Pampa: Detrimental Impacts on Ecosystems and Human Health in a Neglected Biome" Pollutants 3, no. 2: 280-292. https://doi.org/10.3390/pollutants3020020