Impacts of Chlorine on the Change of Chlorophyll Fluorescence Spectrum to Phaeodactylum tricornutum
Abstract
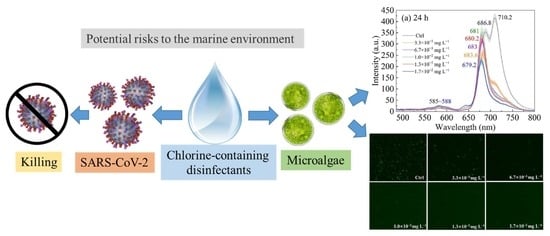
:1. Introduction
2. Materials and Methods
2.1. Microalgae Culture
2.2. Experimental Design
2.3. Measurement of Chlorophyll Fluorescence Spectroscopy
2.4. Statistical Analysis
3. Results and Discussion
3.1. Fluorescence Excitation Spectra
3.2. Fluorescence Emission Spectra
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Facciolà, A.; Laganà, P.; Caruso, G. The COVID-19 pandemic and its implications on the environment. Environ. Res. 2021, 201, 111648. [Google Scholar] [CrossRef]
- García-Ávila, F.; Valdiviezo-Gonzales, L.; Cadme-Galabay, M.; Gutiérrez-Ortega, H.; Altamirano-Cárdenas, L.; Arévalo, C.Z.; Flores del Pino, L. Considerations on water quality and the use of chlorine in times of SARS-CoV-2 (COVID-19) pandemic in the community. Case Stud. Chem. Environ. Eng. 2020, 2, 100049. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, B.; Duan, H.; Liang, C.; Sun, H.; Zhang, J.; Shen, J.; Zhang, L. Key Points of the Program for Disinfection Technology in Special Places during the Coronavirus Disease-2019 (COVID-19) Outbreak. China CDC Wkly. 2020, 2, 140–142. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.; Fang, C.; Deng, Y.; Xu, Z. Intensified Disinfection Amid COVID-19 Pandemic Poses Potential Risks to Water Quality and Safety. Environ. Sci. Technol. 2021, 55, 4084–4086. [Google Scholar] [CrossRef] [PubMed]
- Vannoni, M.; Creach, V.; Barry, J.; Sheahan, D. Chlorine toxicity to Navicula pelliculosa and Achnanthes spp. in a flow-through system: The use of immobilised microalgae and variable chlorophyll fluorescence. Aquat. Toxicol. 2018, 202, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Zhang, D.; Teng, F.; Liao, R.; Cai, Z.; Tao, Y.; Hu, H. Inhibitory effects of ultralow-dose sodium hypochlorite on Microcystis aeruginosa and Chlorella vulgaris: Differences in sensitivity and physiology. Sci. Total Environ. 2021, 774, 145638. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Lu, T.; Zhang, J.; Sun, L.; Hu, B.; Hu, J.; Peñuelas, J.; Zhu, L.; Qian, H. Residual chlorine disrupts the microbial communities and spreads antibiotic resistance in freshwater. J. Hazard. Mater. 2022, 423, 127152. [Google Scholar] [CrossRef]
- Ma, Z.; Gao, K.; Li, W.; Xu, Z.; Lin, H.; Zheng, Y. Impacts of chlorination and heat shocks on growth, pigments and photosynthesis of Phaeodactylum tricornutum (Bacillariophyceae). J. Exp. Mar. Biol. Ecol. 2011, 397, 214–219. [Google Scholar] [CrossRef]
- Garoma, T.; Yazdi, R.E. Investigation of the disruption of algal biomass with chlorine. BMC Plant Biol. 2019, 19, 18. [Google Scholar] [CrossRef]
- Li, N.; Wang, P.; Wang, S.; Wang, C.; Zhou, H.; Kapur, S.; Zhang, J.; Song, Y. Electrostatic charges on microalgae surface: Mechanism and applications. J. Environ. Chem. Eng. 2022, 10, 107516. [Google Scholar] [CrossRef]
- Gan, T.; Zhao, N.; Yin, G.; Chen, M.; Wang, X.; Liu, J.; Liu, W. Optimal chlorophyll fluorescence parameter selection for rapid and sensitive detection of lead toxicity to marine microalgae Nitzschia closterium based on chlorophyll fluorescence technology. J. Photochem. Photobiol. B Biol. 2019, 197, 111551. [Google Scholar] [CrossRef] [PubMed]
- Küpper, H.; Seibert, S.; Parameswaran, A. Fast, Sensitive, and Inexpensive Alternative to Analytical Pigment HPLC: Quantification of Chlorophylls and Carotenoids in Crude Extracts by Fitting with Gauss Peak Spectra. Anal. Chem. 2007, 79, 7611–7627. [Google Scholar] [CrossRef]
- Gruszecki, W.I.; Krupa, Z. Changes of Excitation Spectra of in vivo Chlorophyll Fluorescence during Induction of Photosynthesis. Z. Für Nat. C 1993, 48, 46–51. [Google Scholar] [CrossRef]
- Ebenezer, V.; Ki, J.-S. Physiological and biochemical responses of the marine dinoflagellate Prorocentrum minimum exposed to the oxidizing biocide chlorine. Ecotoxicol. Environ. Saf. 2013, 92, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Sathasivam, R.; Ebenezer, V.; Guo, R.; Ki, J.-S. Physiological and biochemical responses of the freshwater green algae Closterium ehrenbergii to the common disinfectant chlorine. Ecotoxicol. Environ. Saf. 2016, 133, 501–508. [Google Scholar] [CrossRef]
- Patil, J.S.; Jagadeesan, V. Effect of chlorination on the development of marine biofilms dominated by diatoms. Biofouling 2011, 27, 241–254. [Google Scholar] [CrossRef]
- Li, N.; Liu, Z.; Wang, P.; Suman, K.; Zhang, J.; Song, Y. Effects of sodium hypochlorite treatment on the chlorophyll fluorescence in photosystem II of microalgae. Sci. Total Environ. 2022, 833, 155192. [Google Scholar] [CrossRef]
- López-Galindo, C.; Garrido, M.C.; Casanueva, J.F.; Nebot, E. Degradation models and ecotoxicity in marine waters of two antifouling compounds: Sodium hypochlorite and an alkylamine surfactant. Sci. Total Environ. 2010, 408, 1779–1785. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C.; Knapp, M. How to correctly determine the different chlorophyll fluorescence parameters and the chlorophyll fluorescence decrease ratio RFd of leaves with the PAM fluorometer. Photosynthetica 2005, 43, 379–393. [Google Scholar] [CrossRef]
- Berkaloff, C.; Caron, L.; Rousseau, B. Subunit organization of PSI particles from brown algae and diatoms: Polypeptide and pigment analysis. Photosynth. Res. 1990, 23, 181–193. [Google Scholar] [CrossRef]
- Caron, L.; Berkaloff, C. Chlorophyll-Carotenoid Protein Complexes from the Diatom, Phaeodactylum tricornutum: Spectrophotometric, Pigment and Polypeptide Analyses. Plant Cell Physiol. 1987, 28, 775–785. [Google Scholar] [CrossRef]
- Anderson, J.M. Chlorophyll-protein complexes of a Codium species, including a light-harvesting siphonaxanthin-Chlorophylla ab-protein complex, an evolutionary relic of some Chlorophyta. Biochim. Biophys. Acta (BBA) Bioenerg. 1983, 724, 370–380. [Google Scholar] [CrossRef]
- Gao, S.; Chi, Z.; Chen, H.; Zheng, Z.; Weng, Y.; Wang, G. A Supercomplex, of Approximately 720 kDa and Composed of Both Photosystem Reaction Centers, Dissipates Excess Energy by PSI in Green Macroalgae Under Salt Stress. Plant Cell Physiol. 2019, 60, 166–175. [Google Scholar] [CrossRef]
- Pan, X.; Tokutsu, R.; Li, A.; Takizawa, K.; Song, C.; Murata, K.; Yamasaki, T.; Liu, Z.; Minagawa, J.; Li, M. Structural basis of LhcbM5-mediated state transitions in green algae. Nat. Plants 2021, 7, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- da Silva Ferreira, V.; Sant’Anna, C. Impact of culture conditions on the chlorophyll content of microalgae for biotechnological applications. World J. Microbiol. Biotechnol. 2016, 33, 20. [Google Scholar] [CrossRef]
- Aasco, N.C.; Koyama, J.; Imai, S.; Nakamura, K. Toxicity of Residual Chlorines from Hypochlorite-treated Seawater to Marine Amphipod Hyale barbicornis and Estuarine Fish Oryzias javanicus. Water Air Soil Pollut. 2008, 195, 129. [Google Scholar] [CrossRef]
- Wang, J.; Wang, G.; Chen, M.; Wang, Y.; Ding, G.; Zhang, Y.; Kang, Y.; Pan, X. An integrated microfluidic chip for treatment and detection of microalgae cells. Algal Res. 2019, 42, 101593. [Google Scholar] [CrossRef]
- Song, Y.; Li, Z.; Feng, A.; Zhang, J.; Liu, Z.; Li, D. Electrokinetic detection and separation of living algae in a microfluidic chip: Implication for ship’s ballast water analysis. Environ. Sci. Pollut. Res. 2021, 28, 22853–22863. [Google Scholar] [CrossRef] [PubMed]
- Fukuzaki, S. Mechanisms of actions of sodium hypochlorite in cleaning and disinfection processes. Biocontrol Sci. 2006, 11, 147–157. [Google Scholar] [CrossRef]
- Small, D.A.; Chang, W.; Toghrol, F.; Bentley, W.E. Toxicogenomic analysis of sodium hypochlorite antimicrobial mechanisms in Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol. 2007, 74, 176–185. [Google Scholar] [CrossRef]
- Peter, C.; Thoms, S.; Koch, F.; Sartoris, F.J.; Bickmeyer, U. Sponge-derived Ageladine A affects the in vivo fluorescence emission spectra of microalgae. PLoS ONE 2020, 15, e0242464. [Google Scholar] [CrossRef]
- Seder-Colomina, M.; Burgos, A.; Maldonado, J.; Solé, A.; Esteve, I. The effect of copper on different phototrophic microorganisms determined in vivo and at cellular level by confocal laser microscopy. Ecotoxicology 2013, 22, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Sun, C.; Hou, X.; Wu, M.; Yao, Y.; Li, F. Pyrolysis of Arundo donax L. to produce pyrolytic vinegar and its effect on the growth of dinoflagellate Karenia brevis. Bioresour. Technol. 2018, 247, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yao, J.; Duran, R.; Liu, J.; Min, N.; Chen, Z.; Zhu, X.; Zhao, C.; Ma, B.; Pang, W.; et al. Toxic response of the freshwater green algae Chlorella pyrenoidosa to combined effect of flotation reagent butyl xanthate and nickel. Environ. Pollut. 2021, 286, 117285. [Google Scholar] [CrossRef]
- Blatchley, E.R.; John, C.; Brian, P.; Keith, B.; Nicholas, W. The Biological Basis for Ballast Water Performance Standards: “Viable/Non-Viable” or “Live/Dead”? Environ. Sci. Technol. 2018, 52, 8075–8086. [Google Scholar] [CrossRef] [PubMed]





| f/2 Medium | Chemical Medicine | Dosage (g) | |
|---|---|---|---|
| A1 | NaNO3 | 7.5 | |
| A2 | NaH2PO4·2H2O | 0.562 | |
| B | Na2SiO3·9H2O | 3 | |
| C | Na2EDTA | 0.436 | |
| FeCl3·6H2O | 0.315 | ||
| D | CuSO4·5H2O | 0.098 | |
| ZnSO4·7H2O | 0.22 | ||
| CoCl2·6H2O | 0.1 | ||
| MnCl2·6H2O | 1.8 | ||
| Vitamins | |||
| Vitamin B12 | 5 mg | ||
| Vitamin H | 5 mg | ||
| Vitamin B1 | 10 mg | ||
| Ultrapure water | 100 mL | ||
| Culture Time | 24 h | 48 h | ||||||
|---|---|---|---|---|---|---|---|---|
| 439 nm | 467 nm | 439 nm | 467 nm | |||||
| Peak Position | Peak Value | Peak Position | Peak Value | Peak Position | Peak Value | Peak Position | Peak Value | |
| Control | 686.2 | 250.6 ± 12.2 | 686.8 | 352.2 ± 17.4 | 686.4 | 283.7 ± 9.3 | 687 | 341.9 ± 11.1 |
| 3.3 × 10−3 mg L−1 | 683.4 | 292 ± 22.7 | 683.6 | 271.7 ± 19.3 | 684.2 | 88.36 ± 4.3 | 684 | 99.71 ± 4.6 |
| 6.7 × 10−3 mg L−1 | 683.2 | 335.8 ± 12.2 | 683 | 317.9 ± 9.1 | 684.2 | 4.92 ± 0.04 | 684 | 4.36 ± 0.02 |
| 1.0 × 10−2 mg L−1 | 681.6 | 383.3 ± 28.3 | 681 | 370.2 ± 24.2 | 681.2 | 4.37 ± 0.02 | 683.6 | 4.22 ± 0.04 |
| 1.3 × 10−2 mg L−1 | 680 | 411.2 ± 30.1 | 680.2 | 337.2 ± 21.4 | 680.6 | 2.75 ± 0.07 | 680 | 3.07 ± 0.06 |
| 1.7 × 10−2 mg L−1 | 679.2 | 355.2 ± 25.6 | 679.2 | 231.6 ± 13.6 | 680 | 2.45 ± 0.09 | 680 | 2.33 ± 0.1 |
| Culture time | 72 h | 96 h | ||||||
| 439 nm | 467 nm | 439 nm | 467 nm | |||||
| Peak position | Peak value | Peak position | Peak value | Peak position | Peak value | Peak position | Peak value | |
| Control | 687.4 | 333.9 ± 8.8 | 687 | 401.4 ± 10.5 | 686.4 | 375.3 ± 15.0 | 687.6 | 476.1 ± 19.0 |
| 3.3 × 10−3 mg L−1 | 684.4 | 109.8 ± 3.6 | 683.8 | 128.4 ± 4.2 | 684.6 | 96.34 ± 1.8 | 683.6 | 113.3 ± 2.2 |
| 6.7 × 10−3 mg L−1 | 683.2 | 5.51 ± 0.1 | 684 | 6.03 ± 0.1 | 684 | 4.38 ± 0.3 | 684.2 | 4.36 ± 0.2 |
| 1.0 × 10−2 mg L−1 | 683.8 | 4.39 ± 0.1 | 683.2 | 5.13 ± 0.1 | 683.2 | 4.23 ± 0.05 | 684 | 3.81 ± 0.02 |
| 1.3 × 10−2 mg L−1 | 681.8 | 4.20 ± 0.04 | 681 | 5.07 ± 0.03 | 681.8 | 4.02 ± 0.03 | 682.4 | 2.86 ± 0.2 |
| 1.7 × 10−2 mg L−1 | 680.6 | 3.77 ± 0.05 | 680.6 | 5.10 ± 0.06 | 681.8 | 2.38 ± 0.01 | 682.4 | 2.07 ± 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, N.; Chen, S.; Yang, J.; Song, J.; Song, Y. Impacts of Chlorine on the Change of Chlorophyll Fluorescence Spectrum to Phaeodactylum tricornutum. Analytica 2023, 4, 102-112. https://doi.org/10.3390/analytica4020009
Li N, Chen S, Yang J, Song J, Song Y. Impacts of Chlorine on the Change of Chlorophyll Fluorescence Spectrum to Phaeodactylum tricornutum. Analytica. 2023; 4(2):102-112. https://doi.org/10.3390/analytica4020009
Chicago/Turabian StyleLi, Na, Shimeng Chen, Jun Yang, Jun Song, and Yongxin Song. 2023. "Impacts of Chlorine on the Change of Chlorophyll Fluorescence Spectrum to Phaeodactylum tricornutum" Analytica 4, no. 2: 102-112. https://doi.org/10.3390/analytica4020009