Chitosan Nanoparticles as Seed Priming Agents to Alleviate Salinity Stress in Rice (Oryza sativa L.) Seedlings
Abstract
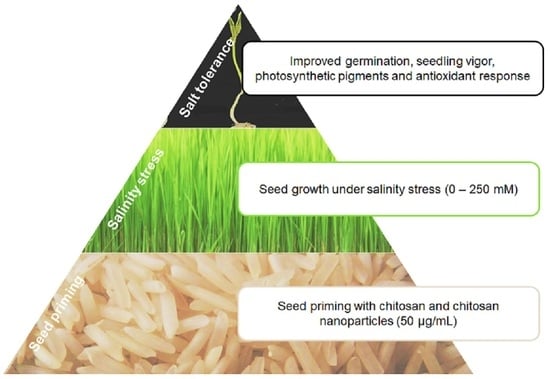
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Chemicals
2.2. Preparation and Characterization of CNPs
2.3. Sterilization, Seed Priming and Growth Conditions
2.4. Measurement of Physiological Indexes
2.5. Enzyme Assays
2.5.1. Catalase
2.5.2. Peroxidase
2.5.3. Superoxide Dismutase
2.6. Estimation of Protein and Carbohydrate Content
2.7. Chlorophyll and Carotenoid Estimation
2.8. Statistical Analysis
3. Results and Discussion
3.1. Preparation and Characterization of CNPs
3.2. Effect of Priming on Germination of Seed and Seedling Vigour
3.3. Chlorophyll and Carotenoid Content
3.4. Antioxidant Enzymes
3.5. Total Protein and Total Reducing Sugar Content
4. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Dimkpa, C.; Deng, C.; Elmer, W.H.; Gardea-Torresdey, J.; White, J.C. Impact of engineered nanomaterials on rice (Oryza sativa L.): A critical review of current knowledge. Environ. Pollut. 2021, 297, 118738. [Google Scholar] [CrossRef] [PubMed]
- Fresco, L. Rice is life. J. Food Compos. Anal. 2005, 4, 249–253. [Google Scholar] [CrossRef]
- Muehe, E.M.; Wang, T.; Kerl, C.F.; Planer-Friedrich, B.; Fendorf, S. Rice production threatened by coupled stresses of climate and soil arsenic. Nat. Commun. 2019, 10, 4985. [Google Scholar] [CrossRef]
- Razzaq, A.; Ali, A.; Safdar, L.B.; Zafar, M.M.; Rui, Y.; Shakeel, A.; Shaukat, A.; Ashraf, M.; Gong, W.; Yuan, Y. Salt stress induces physiochemical alterations in rice grain composition and quality. J. Food Sci. 2020, 85, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Khare, T.; Arya, S.; Shriram, V.; Wani, S.H. Effects of toxic gases, ozone, carbon dioxide, and wastes on plant secondary metabolism. In Medicinal Plants and Environmental Challenges; Springer: Berlin/Heidelberg, Germany, 2017; pp. 81–96. [Google Scholar]
- Chi, W.; Yang, Y.; Zhang, K.; Wang, P.; Du, Y.; Li, X.; Sun, Y.; Liu, T.; Li, F. Seawater intrusion induced cadmium activation via altering its distribution and transformation in paddy soil. Chemosphere 2022, 307, 135805. [Google Scholar] [CrossRef] [PubMed]
- Thu, H.P.T.; Thu, T.N.; Thao, N.D.N.; Le Minh, K.; Do Tan, K. Evaluate the effects of salt stress on physico-chemical characteristics in the germination of rice (Oryza sativa L.) in response to methyl salicylate (MeSA). Biocatal. Agric. Biotechnol. 2020, 23, 101470. [Google Scholar] [CrossRef]
- Kaur, N.; Dhawan, M.; Sharma, I.; Pati, P.K. Interdependency of reactive oxygen species generating and scavenging system in salt sensitive and salt tolerant cultivars of rice. BMC Plant Biol. 2016, 16, 131. [Google Scholar] [CrossRef]
- Liu, C.; Mao, B.; Yuan, D.; Chu, C.; Duan, M. Salt tolerance in rice: Physiological responses and molecular mechanisms. Crop J. 2021, 10, 13–25. [Google Scholar] [CrossRef]
- Pushpalatha, G.; Harish Kumar, G. Gene expression analysis reveals diversified responsiveness to salt stress in rice genotypes. Indian J. Plant Physiol. 2018, 23, 833–843. [Google Scholar] [CrossRef]
- Math, S.; Arya, S.; Sonawane, H.; Patil, V.; Chaskar, M. Arbuscular mycorrhizal (Glomus fasciculatum) fungi as a plant immunity booster against fungal pathogen. Curr. Agric. Res. J. 2019, 7, 99–107. [Google Scholar] [CrossRef]
- Sashidhar, P.; Arya, S.S.; Das, R.K.; Dubey, M.K.; Lenka, S.K. Nanobiotechnology for plant genome engineering and crop protection. In Genetically Modified Crops in Asia Pacific; CSIRO: Australia, 2021; pp. 279–310. [Google Scholar]
- Arya, S.S.; Tanwar, N.; Lenka, S.K. Prospects of nano-and peptide-carriers to deliver CRISPR cargos in plants to edit across and beyond central dogma. Nanotechnol. Environ. Eng. 2021, 6, 22. [Google Scholar] [CrossRef]
- Zafar, S.; Perveen, S.; Kamran Khan, M.; Shaheen, M.R.; Hussain, R.; Sarwar, N.; Rashid, S.; Nafees, M.; Farid, G.; Alamri, S. Effect of zinc nanoparticles seed priming and foliar application on the growth and physio-biochemical indices of spinach (Spinacia oleracea L.) under salt stress. PLoS ONE 2022, 17, e0263194. [Google Scholar] [CrossRef]
- Arya, S.S.; Lenka, S.K.; Cahill, D.M.; Rookes, J.E. Designer nanoparticles for plant cell culture systems: Mechanisms of elicitation and harnessing of specialized metabolites. BioEssays 2021, 43, 2100081. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, H.; Arya, S.; Math, S.; Shelke, D. Myco-synthesized silver and titanium oxide nanoparticles as seed priming agents to promote seed germination and seedling growth of Solanum lycopersicum: A comparative study. Int. Nano Lett. 2021, 11, 371–379. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, C.; Rawat, S.; Cota-Ruiz, K.; Medina-Velo, I.; Gardea-Torresdey, J.L. Evaluation of the effects of nanomaterials on rice (Oryza sativa L.) responses: Underlining the benefits of nanotechnology for agricultural applications. ACS Agric. Sci. Technol. 2021, 1, 44–54. [Google Scholar] [CrossRef]
- Balusamy, S.R.; Rahimi, S.; Sukweenadhi, J.; Sunderraj, S.; Shanmugam, R.; Thangavelu, L.; Mijakovic, I.; Perumalsamy, H. Chitosan, chitosan nanoparticles and modified chitosan biomaterials, a potential tool to combat salinity stress in plants. Carbohydr. Polym. 2022, 284, 119189. [Google Scholar] [CrossRef]
- Arya, S.S.; Mahto, B.K.; Ramkumar, T.R.; Lenka, S.K. Sharpening gene editing toolbox in Arabidopsis for plants. J. Plant Biochem. Biotechnol. 2020, 29, 769–784. [Google Scholar] [CrossRef]
- Arya, S.S.; Rookes, J.E.; Cahill, D.M.; Lenka, S.K. Chitosan nanoparticles and their combination with methyl jasmonate for the elicitation of phenolics and flavonoids in plant cell suspension cultures. Int. J. Biol. Macromol. 2022, 214, 632–641. [Google Scholar] [CrossRef]
- Shah, B.R.; Li, Y.; Jin, W.; An, Y.; He, L.; Li, Z.; Xu, W.; Li, B. Preparation and optimization of Pickering emulsion stabilized by chitosan-tripolyphosphate nanoparticles for curcumin encapsulation. Food Hydrocoll. 2016, 52, 369–377. [Google Scholar] [CrossRef]
- Jafari, Z.; Rad, A.S.; Baharfar, R.; Asghari, S.; Esfahani, M.R. Synthesis and application of chitosan/tripolyphosphate/graphene oxide hydrogel as a new drug delivery system for Sumatriptan Succinate. J. Mol. Liq. 2020, 315, 113835. [Google Scholar] [CrossRef]
- Mathew, S.A.; Praveena, P.; Dhanavel, S.; Manikandan, R.; Senthilkumar, S.; Stephen, A. Luminescent chitosan/carbon dots as an effective nano-drug carrier for neurodegenerative diseases. RSC Adv. 2020, 10, 24386–24396. [Google Scholar] [CrossRef]
- Kashyap, P.L.; Xiang, X.; Heiden, P. Chitosan nanoparticle based delivery systems for sustainable agriculture. Int. J. Biol. Macromol. 2015, 77, 36–51. [Google Scholar] [CrossRef]
- Li, R.; He, J.; Xie, H.; Wang, W.; Bose, S.K.; Sun, Y.; Hu, J.; Yin, H. Effects of chitosan nanoparticles on seed germination and seedling growth of wheat (Triticum aestivum L.). Int. J. Biol. Macromol. 2019, 126, 91–100. [Google Scholar] [CrossRef]
- Divya, K.; Vijayan, S.; Nair, S.J.; Jisha, M. Optimization of chitosan nanoparticle synthesis and its potential application as germination elicitor of Oryza sativa L. Int. J. Biol. Macromol. 2019, 124, 1053–1059. [Google Scholar] [CrossRef]
- Shangari, N.; O’Brien, P.J. Catalase activity assays. Curr. Protoc. Toxicol. 2006, 27, 7.7.1–7.7.16. [Google Scholar] [CrossRef] [PubMed]
- Hadwan, M.H. Simple spectrophotometric assay for measuring catalase activity in biological tissues. BMC Biochem. 2018, 19, 7. [Google Scholar] [CrossRef] [PubMed]
- Goldblith, S.A.; Proctor, B.E. Photometric determination of catalase activity. J. Biol. Chem. 1950, 187, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Lurie, S.; Fallik, E.; Handros, A.; Shapira, R. The possible involvement of peroxidase in resistance toBotrytis cinereain heat treated tomato fruit. Physiol. Mol. Plant Pathol. 1997, 50, 141–149. [Google Scholar] [CrossRef]
- Stewart, R.R.; Bewley, J.D. Lipid peroxidation associated with accelerated aging of soybean axes. Plant Physiol. 1980, 65, 245–248. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Salah, S.M.; Yajing, G.; Dongdong, C.; Jie, L.; Aamir, N.; Qijuan, H.; Weimin, H.; Mingyu, N.; Jin, H. Seed priming with polyethylene glycol regulating the physiological and molecular mechanism in rice (Oryza sativa L.) under nano-ZnO stress. Sci. Rep. 2015, 5, srep14278. [Google Scholar] [CrossRef]
- Santo Pereira, A.d.E.; Oliveira, H.C.; Fraceto, L.F. Polymeric nanoparticles as an alternative for application of gibberellic acid in sustainable agriculture: A field study. Sci. Rep. 2019, 9, 7135. [Google Scholar] [CrossRef]
- Tang, X.; Mu, X.; Shao, H.; Wang, H.; Brestic, M. Global plant-responding mechanisms to salt stress: Physiological and molecular levels and implications in biotechnology. Crit. Rev. Biotechnol. 2015, 35, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.N.D.; Ribeiro, R.V.; Ferreira-Silva, S.L.; Viégas, R.A.; Silveira, J.A.G. Salt stress induced damages on the photosynthesis of physic nut young plants. Sci. Agric. 2011, 68, 62–68. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Q.; Song, H.; Rong, X.; Abdelbagi, M.I. Responses of different rice (Oryza sativa L.) genotypes to salt stress and relation to carbohydrate metabolism and chlorophyll content. Afr. J. Agric. Res. 2012, 7, 19–27. [Google Scholar]
- Ozturk, L.; Demir, Y.; Unlukara, A.; Karatas, I.; Kurunc, A.; Duzdemir, O. Effects of long-term salt stress on antioxidant system, chlorophyll and proline contents in pea leaves. Rom. Biotechnol. Lett. 2012, 17, 7227–7236. [Google Scholar]
- Mekawy, A.M.M.; Abdelaziz, M.N.; Ueda, A. Apigenin pretreatment enhances growth and salinity tolerance of rice seedlings. Plant Physiol. Biochem. 2018, 130, 94–104. [Google Scholar] [CrossRef]
- Ramel, F.; Birtic, S.; Ginies, C.; Soubigou-Taconnat, L.; Triantaphylidès, C.; Havaux, M. Carotenoid oxidation products are stress signals that mediate gene responses to singlet oxygen in plants. Proc. Natl. Acad. Sci. USA 2012, 109, 5535–5540. [Google Scholar] [CrossRef]
- Moharekar, S.; Lokhande, S.; Hara, T.; Tanaka, R.; Tanaka, A.; Chavan, P. Effect of salicylic acid on chlorophyll and carotenoid contents of wheat and moong seedlings. Photosynthetica 2003, 41, 315–317. [Google Scholar] [CrossRef]
- Sen, S.K.; Chouhan, D.; Das, D.; Ghosh, R.; Mandal, P. Improvisation of salinity stress response in mung bean through solid matrix priming with normal and nano-sized chitosan. Int. J. Biol. Macromol. 2020, 145, 108–123. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziz, H. Effect of priming with chitosan nanoparticles on germination, seedling growth and antioxidant enzymes of broad beans. Catrina: Int. J. Environ. Sci. 2019, 18, 81–86. [Google Scholar] [CrossRef]
- Guan, Y.-J.; Hu, J.; Wang, X.-J.; Shao, C.-X. Seed priming with chitosan improves maize germination and seedling growth in relation to physiological changes under low temperature stress. J. Zhejiang Univ. Sci. B 2009, 10, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Agbodjato, N.A.; Noumavo, P.A.; Adjanohoun, A.; Agbessi, L.; Baba-Moussa, L. Synergistic effects of plant growth promoting rhizobacteria and chitosan on in vitro seeds germination, greenhouse growth, and nutrient uptake of maize (Zea mays L.). Biotechnol. Res. Int. 2016, 2016, 7830182. [Google Scholar] [CrossRef]
- Manjunatha, G.; Roopa, K.; Prashanth, G.N.; Shekar Shetty, H. Chitosan enhances disease resistance in pearl millet against downy mildew caused by Sclerospora graminicola and defence-related enzyme activation. Pest Manag. Sci. Former. Pestic. Sci. 2008, 64, 1250–1257. [Google Scholar] [CrossRef]
- Nakasato, D.Y.; Pereira, A.E.; Oliveira, J.L.; Oliveira, H.C.; Fraceto, L.F. Evaluation of the effects of polymeric chitosan/tripolyphosphate and solid lipid nanoparticles on germination of Zea mays, Brassica rapa and Pisum sativum. Ecotoxicol. Environ. Saf. 2017, 142, 369–374. [Google Scholar] [CrossRef]
- Songlin, R.; Qingzhong, X. Effects of chitosan coating on seed germination and salt-tolerance of seedling in hybrid rice (Oryza sativa L.). Zuo Wu Xue Bao 2002, 28, 803–808. [Google Scholar]
- Siddaiah, C.N.; Prasanth, K.V.H.; Satyanarayana, N.R.; Mudili, V.; Gupta, V.K.; Kalagatur, N.K.; Satyavati, T.; Dai, X.-F.; Chen, J.-Y.; Mocan, A. Chitosan nanoparticles having higher degree of acetylation induce resistance against pearl millet downy mildew through nitric oxide generation. Sci. Rep. 2018, 8, 2485. [Google Scholar] [CrossRef]
- Zayed, M.; Elkafafi, S.; Zedan, A.M.; Dawoud, S.F. Effect of nano chitosan on growth, physiological and biochemical parameters of Phaseolus vulgaris under salt stress. J. Plant Prod. 2017, 8, 577–585. [Google Scholar] [CrossRef]
- Kananont, N.; Pichyangkura, R.; Chanprame, S.; Chadchawan, S.; Limpanavech, P. Chitosan specificity for the in vitro seed germination of two Dendrobium orchids (Asparagales: Orchidaceae). Sci. Hortic. 2010, 124, 239–247. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, W.; Yin, H.; Zhao, X.; Du, Y. Oligochitosan induces programmed cell death in tobacco suspension cells. Carbohydr. Polym. 2012, 87, 2270–2278. [Google Scholar] [CrossRef]
- Mazancová, P.; Némethová, V.; Treľová, D.; Kleščíková, L.; Lacík, I.; Rázga, F. Dissociation of chitosan/tripolyphosphate complexes into separate components upon pH elevation. Carbohydr. Polym. 2018, 192, 104–110. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soni, A.T.; Rookes, J.E.; Arya, S.S. Chitosan Nanoparticles as Seed Priming Agents to Alleviate Salinity Stress in Rice (Oryza sativa L.) Seedlings. Polysaccharides 2023, 4, 129-141. https://doi.org/10.3390/polysaccharides4020010
Soni AT, Rookes JE, Arya SS. Chitosan Nanoparticles as Seed Priming Agents to Alleviate Salinity Stress in Rice (Oryza sativa L.) Seedlings. Polysaccharides. 2023; 4(2):129-141. https://doi.org/10.3390/polysaccharides4020010
Chicago/Turabian StyleSoni, Akanksha T., James E. Rookes, and Sagar S. Arya. 2023. "Chitosan Nanoparticles as Seed Priming Agents to Alleviate Salinity Stress in Rice (Oryza sativa L.) Seedlings" Polysaccharides 4, no. 2: 129-141. https://doi.org/10.3390/polysaccharides4020010