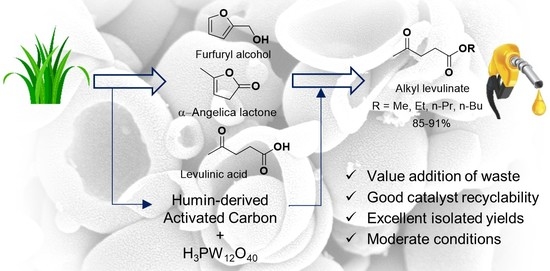
Production of Alkyl Levulinates from Carbohydrate-Derived Chemical Intermediates Using Phosphotungstic Acid Supported on Humin-Derived Activated Carbon (PTA/HAC) as a Recyclable Heterogeneous Acid Catalyst
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Humin
2.3. Preparation of HAC
2.4. Preparation of PTA/HAC-600 Catalyst
2.5. Characterization Methods of Catalyst
2.6. Catalytic Conversion of Biomass-Derived FAL, LA, and α-AGL to ALs
3. Results
3.1. Physicochemical Characterization
3.2. Catalytic Tests
3.3. Catalyst Recyclability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| α-AGL | α-Angelica lactone |
| HAC | Humin-derived activated carbon |
| AL | Alkyl levulinate |
| ML | Methyl levulinate |
| LA | Levulinic acid |
| FF | Furfural |
| FAL | Furfuryl alcohol |
| EL | Ethyl levulinate |
| PL | Propyl levulinate |
| BL | Butyl levulinate |
| HPA | Heteropoly acid |
| PTA | Phosphotungstic acid |
References
- Ubando, A.T.; Felix, C.B.; Chen, W.-H. Biorefineries in Circular Bioeconomy: A Comprehensive Review. Bioresour. Technol. 2020, 299, 122585. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Feng, Y.; Fu, J.; Guo, H.; Guo, Y.; Han, B.; Jiang, Z.; Kong, L.; Li, C.; Liu, H.; et al. Catalytic Conversion of Lignocellulosic Biomass into Chemicals and Fuels. Green Energy Environ. 2023, 8, 10–114. [Google Scholar] [CrossRef]
- Alonso, D.M.; Bond, J.Q.; Dumesic, J.A. Catalytic Conversion of Biomass to Biofuels. Green Chem. 2010, 12, 1493–1513. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G. Composition, Properties and Challenges of Algae Biomass for Biofuel Application: An Overview. Fuel 2016, 181, 1–33. [Google Scholar] [CrossRef]
- Jérôme, F.; De Oliveira Vigier, K. Catalytic Conversion of Carbohydrates to Furanic Derivatives in the Presence of Choline Chloride. Catalysts 2017, 7, 218. [Google Scholar] [CrossRef] [Green Version]
- Bhaumik, P.; Dhepe, P.L. Solid Acid Catalyzed Synthesis of Furans from Carbohydrates. Catal. Rev. 2016, 58, 36–112. [Google Scholar] [CrossRef]
- Zhao, Y.; Lu, K.; Xu, H.; Zhu, L.; Wang, S. A Critical Review of Recent Advances in the Production of Furfural and 5-Hydroxymethylfurfural from Lignocellulosic Biomass through Homogeneous Catalytic Hydrothermal Conversion. Renew. Sustain. Energy Rev. 2021, 139, 110706. [Google Scholar] [CrossRef]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; Granados, M.L. Furfural: A Renewable and Versatile Platform Molecule for the Synthesis of Chemicals and Fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- Eseyin, A.E.; Steele, P.H. An Overview of the Applications of Furfural and Its Derivatives. Int. J. Adv. Chem. 2015, 3, 42–47. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Paone, E.; Rodríguez-Padrón, D.; Luque, R.; Mauriello, F. Recent Catalytic Routes for the Preparation and the Upgrading of Biomass Derived Furfural and 5-Hydroxymethylfurfural. Chem. Soc. Rev. 2020, 49, 4273–4306. [Google Scholar] [CrossRef]
- Dusselier, M.; Mascal, M.; Sels, B.F. Top Chemical Opportunities from Carbohydrate Biomass: A Chemist’s View of the Biorefinery. In Selective Catalysis for Renewable Feedstocks and Chemicals; Nicholas, K.M., Ed.; Topics in Current Chemistry; Springer International Publishing: Cham, Switzerland, 2014; Volume 353, pp. 1–40. ISBN 978-3-319-08653-8. [Google Scholar]
- Tian, Y.; Zhang, F.; Wang, J.; Cao, L.; Han, Q. A Review on Solid Acid Catalysis for Sustainable Production of Levulinic Acid and Levulinate Esters from Biomass Derivatives. Bioresour. Technol. 2021, 342, 125977. [Google Scholar] [CrossRef]
- Dutta, S.; Bhat, N.S. Recent Advances in the Value Addition of Biomass-Derived Levulinic Acid: A Review Focusing on Its Chemical Reactivity Patterns. ChemCatChem 2021, 13, 3202–3222. [Google Scholar] [CrossRef]
- Hayes, D.J.; Fitzpatrick, S.; Hayes, M.H.B.; Ross, J.R.H. The Biofine Process– Production of Levulinic Acid, Furfural, and Formic Acid from Lignocellulosic Feedstocks. In Biorefineries-Industrial Processes and Products; Kamm, B., Gruber, P.R., Kamm, M., Eds.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2005; pp. 139–164. ISBN 978-3-527-61984-9. [Google Scholar]
- Di Bucchianico, D.D.M.; Wang, Y.; Buvat, J.-C.; Pan, Y.; Moreno, V.C.; Leveneur, S. Production of Levulinic Acid and Alkyl Levulinates: A Process Insight. Green Chem. 2022, 24, 614–646. [Google Scholar] [CrossRef]
- Démolis, A.; Essayem, N.; Rataboul, F. Synthesis and Applications of Alkyl Levulinates. ACS Sustain. Chem. Eng. 2014, 2, 1338–1352. [Google Scholar] [CrossRef]
- Ahmad, K.A.; Siddiqui, M.H.; Alam, M.I.; Haider, M.A.; Ahmad, E. Keggin Heteropolyacid Catalysts: Synthesis, Heterogenization, and Application in Conversion of Biomass-Derived Molecules. In Catalysis; Spivey, J., Han, Y.-F., Shekhawat, D., Eds.; Royal Society of Chemistry: Cambridge, UK, 2022; Volume 34, pp. 206–247. ISBN 978-1-83916-499-6. [Google Scholar]
- Zhang, Q.; Luo, Q.; Wu, Y.; Yu, R.; Cheng, J.; Zhang, Y. Construction of a Keggin Heteropolyacid/Ni-MOF Catalyst for Esterification of Fatty Acids. RSC Adv. 2021, 11, 33416–33424. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Zhou, Z.; Wang, M.; Shang, N.; Gao, W.; Cheng, X.; Gao, S.; Gao, Y.; Wang, C. Highly Dispersed Pd Anchored on Heteropolyacid Modified ZrO2 for High Efficient Hydrodeoxygenation of Lignin-Derivatives. Fuel 2023, 334, 126768. [Google Scholar] [CrossRef]
- Luo, X.; Wu, H.; Li, C.; Li, Z.; Li, H.; Zhang, H.; Li, Y.; Su, Y.; Yang, S. Heteropoly Acid-Based Catalysts for Hydrolytic Depolymerization of Cellulosic Biomass. Front. Chem. 2020, 8, 580146. [Google Scholar] [CrossRef]
- Bhat, N.S.; Mal, S.S.; Dutta, S. Recent Advances in the Preparation of Levulinic Esters from Biomass-Derived Furanic and Levulinic Chemical Platforms Using Heteropoly Acid (HPA) Catalysts. Mol. Catal. 2021, 505, 111484. [Google Scholar] [CrossRef]
- Wu, Y.; Ye, X.; Yang, X.; Wang, X.; Chu, W.; Hu, Y. Heterogenization of Heteropolyacids: A General Discussion on the Preparation of Supported Acid Catalysts. Ind. Eng. Chem. Res. 1996, 35, 2546–2560. [Google Scholar] [CrossRef]
- Lam, E.; Luong, J.H.T. Carbon Materials as Catalyst Supports and Catalysts in the Transformation of Biomass to Fuels and Chemicals. ACS Catal. 2014, 4, 3393–3410. [Google Scholar] [CrossRef]
- Lin, Y.; Yu, J.; Zhang, X.; Fang, J.; Lu, G.-P.; Huang, H. Carbohydrate-Derived Porous Carbon Materials: An Ideal Platform for Green Organic Synthesis. Chin. Chem. Lett. 2022, 33, 186–196. [Google Scholar] [CrossRef]
- Ayashi, N.; Chermahini, A.N.; Saraji, M. Biomass Conversion to Alkyl Levulinates Using Heteropoly Acid Carbon Mesoporous Composites. Process Saf. Environ. Prot. 2022, 160, 988–1000. [Google Scholar] [CrossRef]
- Chhabra, T.; Rohilla, J.; Krishnan, V. Nanoarchitectonics of Phosphomolybdic Acid Supported on Activated Charcoal for Selective Conversion of Furfuryl Alcohol and Levulinic Acid to Alkyl Levulinates. Mol. Catal. 2022, 519, 112135. [Google Scholar] [CrossRef]
- Patil, S.K.R.; Heltzel, J.; Lund, C.R.F. Comparison of Structural Features of Humins Formed Catalytically from Glucose, Fructose, and 5-Hydroxymethylfurfuraldehyde. Energy Fuels 2012, 26, 5281–5293. [Google Scholar] [CrossRef]
- Xu, Z.; Yang, Y.; Yan, P.; Xia, Z.; Liu, X.; Zhang, Z.C. Mechanistic Understanding of Humin Formation in the Conversion of Glucose and Fructose to 5-Hydroxymethylfurfural in [BMIM]Cl Ionic Liquid. RSC Adv. 2020, 10, 34732–34737. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhu, Y.; Liao, Y.; Wang, H.; Liu, Q.; Ma, L.; Wang, C. Advances in Understanding the Humins: Formation, Prevention and Application. Appl. Energy Combust. Sci. 2022, 10, 100062. [Google Scholar] [CrossRef]
- Yang, J.; Niu, X.; Wu, H.; Zhang, H.; Ao, Z.; Zhang, S. Valorization of Humin as a Glucose Derivative to Fabricate a Porous Carbon Catalyst for Esterification and Hydroxyalkylation/Alkylation. Waste Manag. 2020, 103, 407–415. [Google Scholar] [CrossRef]
- Bhat, N.S.; Vinod, N.; Onkarappa, S.B.; Dutta, S. Hydrochloric Acid-Catalyzed Coproduction of Furfural and 5-(Chloromethyl)furfural Assisted by a Phase Transfer Catalyst. Carbohydr. Res. 2020, 496, 108105. [Google Scholar] [CrossRef]
- Kozhevnikov, I.V.; Kloetstra, K.R.; Sinnema, A.; Zandbergen, H.W.; van Bekkum, H. Study of Catalysts Comprising Heteropoly Acid H3PW12O40 Supported on MCM-41 Molecular Sieve and Amorphous Silica. J. Mol. Catal. A Chem. 1996, 114, 287–298. [Google Scholar] [CrossRef]
- ASTM D4607-14R21; Standard Test Method for Determination of Iodine Number of Activated Carbon. ASTM International: West Conshohocken, PA, USA, 2021. [CrossRef]









| Sample | IN (mg/g) | SBET (m2/g) | Vtotal (cc/g) | Davg (nm) |
|---|---|---|---|---|
| HAC-500 | 727 | 1203 | 0.32 | 3.4 |
| HAC-600 | 853 | 1425 | 0.33 | 2.8 |
| 20%PTA/HAC-500 | - | 937 | 0.11 | 3.4 |
| 20%PTA/HAC-600 | - | 947 | 0.12 | 2.5 |
| Entry | Product | Yield (%) |
|---|---|---|
| 1 | ML | 87 |
| 2 | EL | 85 |
| 3 | PL | 83 |
| 4 | BL | 85 |
| Entry | Product | Yield (%) |
|---|---|---|
| 1 | ML | 85 |
| 2 | EL | 88 |
| 3 | PL | 90 |
| 4 | BL | 87 |
| Entry | Product | Yield (%) |
|---|---|---|
| 1 | ML | 91 |
| 2 | EL | 84 |
| 3 | PL | 86 |
| 4 | BL | 88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinod, N.; Dutta, S. Production of Alkyl Levulinates from Carbohydrate-Derived Chemical Intermediates Using Phosphotungstic Acid Supported on Humin-Derived Activated Carbon (PTA/HAC) as a Recyclable Heterogeneous Acid Catalyst. Chemistry 2023, 5, 800-812. https://doi.org/10.3390/chemistry5020057
Vinod N, Dutta S. Production of Alkyl Levulinates from Carbohydrate-Derived Chemical Intermediates Using Phosphotungstic Acid Supported on Humin-Derived Activated Carbon (PTA/HAC) as a Recyclable Heterogeneous Acid Catalyst. Chemistry. 2023; 5(2):800-812. https://doi.org/10.3390/chemistry5020057
Chicago/Turabian StyleVinod, Nivedha, and Saikat Dutta. 2023. "Production of Alkyl Levulinates from Carbohydrate-Derived Chemical Intermediates Using Phosphotungstic Acid Supported on Humin-Derived Activated Carbon (PTA/HAC) as a Recyclable Heterogeneous Acid Catalyst" Chemistry 5, no. 2: 800-812. https://doi.org/10.3390/chemistry5020057