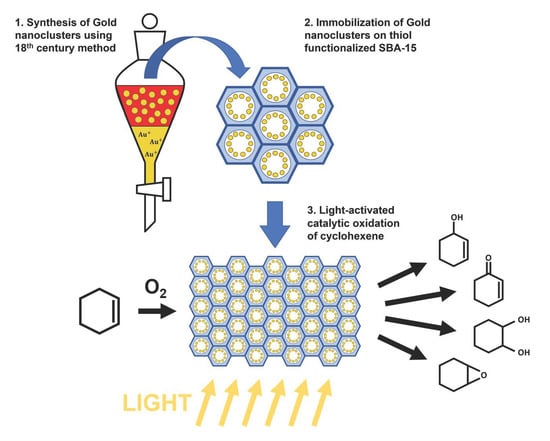
Gold Clusters Immobilized by Post-Synthesis Methods on Thiol-Containing SBA-15 Mesoporous Materials for the Aerobic Oxidation of Cyclohexene: Influence of Light and Hydroperoxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Functionalization of Plate-like SBA-15 with 3-Mercaptopropyltrimethoxysilane (MPTMS)
2.2. Synthesis of the Au NCs
2.3. Immobilization of the Au NCs on the Functionalized SBA-15
2.4. Catalytic Tests
2.5. Characterization Techniques
3. Results and Discussion
3.1. Characterization of the Materials
3.1.1. SBA-15 Functionalized with Thiol Groups
3.1.2. Gold-Containing Catalysts
3.2. Catalytic Tests
3.2.1. Tests at Ambient Lighting in Presence of TBHP
3.2.2. Tests Either in Darkness or in Absence of TBHP
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Haruta, M.; Kobayashi, T.; Sano, H.; Yamada, N. Novel gold catalysts for the oxidation of carbon monoxide at a temperature far below 0 °C. Chem. Let. 1987, 16, 405–408. [Google Scholar] [CrossRef] [Green Version]
- Hutchings, G.J. Vapor phase hydrochlorination of ethylene: Correlation of catalytic activity of supported metal chloride catalysts. J. Catal. 1985, 96, 292–295. [Google Scholar] [CrossRef]
- Bond, G. The Early History of Catalysis by Gold. A review of the literature before 1978. Gold Bull. 2008, 41, 235–241. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Chen, W. Sub-nanometre sized metal clusters: From synthetic challenges to the unique property discoveries. Chem. Soc. Rev. 2012, 41, 3595–3623. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Concepcion, P.; Boronat, M.; Sabater, M.J.; Navas, J.; Yacaman, J.M.; Larios, E.; Posadas, A.; López-Quintela, M.A.; Buceta, D.; et al. Exceptional oxidation activity with size-controlled supported gold clusters of low atomicity. Nat. Chem. 2013, 5, 775–781. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Jin, R. Heterogeneous catalysis by gold and gold-based bimetal nanoclusters. Nanotoday 2018, 18, 86–102. [Google Scholar] [CrossRef]
- Li, G.; Jin, R. Atomically Precise Gold Nanoclusters as New Model Catalysts. Acc. Chem. Res. 2013, 46, 1749–1758. [Google Scholar] [CrossRef]
- Hashmi, A.S.K.; Hutchings, G.J. Gold Catalysis. Angew. Chem. Int. Ed. 2006, 45, 7896–7936. [Google Scholar] [CrossRef]
- Corma, A.; García, H. Supported gold nanoparticles as catalysts for organic reactions. Chem. Soc. Rev. 2008, 37, 2096–2126. [Google Scholar] [CrossRef]
- Patil, N.T. Heterogeneous—Acid Catalysis with Metal Nanoparticles. ChemCatChem 2011, 3, 1121–1125. [Google Scholar] [CrossRef]
- Hendrich, C.M.; Sekine, K.; Koshikawa, T.; Tanaka, K.; Hashmi, A.S.K. Homogeneous and heterogeneous gold catalysis for materials science. Chem. Rev. 2021, 121, 9113–9163. [Google Scholar] [CrossRef]
- Sankar, M.; He, Q.; Engel, R.V.; Sainna, M.A.; Logsdail, A.J.; Roldan, A.; Willock, D.J.; Agarwal, N.; Kiely, C.J.; Hutchings, G.J. Role of the Support in Gold-Containing Nanoparticles as Heterogeneous Catalysts. Chem. Rev. 2020, 120, 3890–3938. [Google Scholar] [CrossRef] [Green Version]
- Lémery, N. Cours de Chymie; Herissant: Paris, France, 1756. [Google Scholar]
- Mayoral, A.; Agúndez, J.; Pascual-Valderrama, I.-M.; Pérez-Pariente, J. Generation of gold nanoparticles according to procedures described in the eighteenth century. Gold Bull. 2014, 47, 161–165. [Google Scholar] [CrossRef] [Green Version]
- Agundez, J.; Martín, L.; Mayoral, A.; Pérez-Pariente, J. Gold nanoclusters prepared from an eighteenth century two-phases procedure supported on thiol-containing SBA-15 for liquid phase oxidation of cyclohexene with molecular oxygen. Catal. Today 2018, 304, 172–180. [Google Scholar] [CrossRef]
- Agúndez, J.; Ares, C.; Márquez-Álvarez, C.; Pérez-Pariente, J. Catalytic oxidation of cyclohexene by supported gold nanoclusters synthesized in a two-liquid phases system containing eucalyptus essential oil. Mol. Catal. 2020, 488, 110922. [Google Scholar] [CrossRef]
- Mato, A.; Agúndez, J.; Márquez-Álvarez, C.; Mayoral, A.; Pérez-Pariente, J. Modulation of the Activity of Gold Clusters Immobilized on Functionalized Mesoporous Materials in the Oxidation of Cyclohexene via the Functional Group. The Case of Aminopropyl Moiety. Molecules 2020, 25, 5756. [Google Scholar] [CrossRef]
- De la Serna Valdés, R.; Agúndez, J.; Márquez-Álvarez, C.; Pérez-Pariente, J. Immobilization of gold on short-channel mesoporous SBA-15 functionalized with thiol and hydrophobic groups for oxidation reactions. Catal. Today 2020, 354, 77–89. [Google Scholar] [CrossRef]
- Wang, C.; Astruc, D. Nanogold plasmonic photocatalysis for organic synthesis and clean energy conversion. Chem. Soc. Rev. 2014, 43, 7188–7216. [Google Scholar] [CrossRef] [Green Version]
- Kale, M.J.; Avanesian, T.; Christopher, P. Direct Photocatalysis by Plasmonic Nanostructures. ACS Catal. 2014, 4, 116–128. [Google Scholar] [CrossRef]
- Chen, H.; Liu, C.; Wang, M.; Zhang, C.; Luo, N.; Wang, Y.; Abroshan, H.; Li, G.; Wang, F. Visible Light Gold Nanocluster Photocatalyst: Selective Aerobic Oxidation of amines to Imines. ACS Catal. 2017, 7, 3632–3638. [Google Scholar] [CrossRef]
- Tian, S.; Cao, Y.; Chen, T.; Zang, S.; Xie, J. Ligand-protected atomically precise gold nanoclusters as model catalysts for oxidation reactions. Chem. Commun. 2020, 56, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.D.; Xu, Y.-J.; Jenkins, P.; MacMorn, P.; Landon, P.; Enache, D.I.; Carley, A.F.; Attard, G.A.; Hutchings, J.G.; King, F.; et al. Tunable gold catalysts for selective hydrocarbon oxidation under mild conditions. Nature 2005, 436, 1132–1135. [Google Scholar] [CrossRef] [PubMed]
- Margolese, D.; Melero, J.A.; Christiansen, S.C.; Chmelka, B.F.; Stuckey, G.C. Direct Syntheses of Ordered SBA-15 Mesoporous silica Containing Sulfonic Acid Groups. Chem. Mater. 2000, 12, 2448–2459. [Google Scholar] [CrossRef]
- Cano-Serrano, E.; Blanco-Brieva, G.; Campos-Martin, J.M.; Fierro, J.L.G. Acid-functionalized amorphous silica by chemical grafting-quantitative oxidation of thiol groups. Langmuir 2003, 19, 7621–7627. [Google Scholar] [CrossRef]
- Nasef, M.M.; Saidi, H. Surface studies of radiation grafted sulfonic acid membranes: XPS and SEM analysis. Appl. Surf. Sci. 2006, 252, 3073–3084. [Google Scholar] [CrossRef]
- Woehrle, G.H.; Warner, M.G.; Hutchison, J.E. Ligand exchange reactions yield subnanometer, thiol-stabilized gold particles with defined optical transitions. J. Phys. Chem. B 2002, 106, 9979–9981. [Google Scholar] [CrossRef]
- Rodríguez-Vázquez, M.J.; Blanco, M.C.; Lourido, R.; López-Quintela, M.A. Synthesis of atomic gold clusters with strong electrocatalytic activities. Langmuir 2008, 24, 12690–12694. [Google Scholar]
- Lugo, G.; Schwanen, V.; Fresch, B.; Remacle, F. Charge redistribution effects on the UV-Vis spectra of small ligated gold clusters: A computational study. J. Phys. Chem. C 2015, 119, 10969–10980. [Google Scholar] [CrossRef]
- Bogdanchikova, N.; Pestryakov, A.; Tuzovskaya, I.; Zepeda, T.A.; Farias, M.H.; Tiznado, H.; Martynyuk, O.A. Effect of redox treatments on activation and deactivation of gold nanospecies supported on mesoporous silica in CO oxidation. Fuel 2013, 110, 40–47. [Google Scholar] [CrossRef]
- Bourg, M.-C.; Badia, A.; Lennox, R.B. Gold-Sulfur Bonding in 2D and 3D Self-Assembled Monolayers: XPS Characterization. J. Phys. Chem. B 2000, 104, 6562–6567. [Google Scholar] [CrossRef]
- Peters, S.; Peredko, S.; Neeb, M.; Eberhardt, W.; Al-Hada, M. Size-dependent XPS spectra of small supported Au-clusters. Surf. Sci. 2013, 608, 129–134. [Google Scholar] [CrossRef]
- Tal, A.A.; Olovsson, W.; Abrikosov, I.A. Origin of the core-level binding energy shifts in Au nanoclusters. Phys. Rev. B. 2017, 95, 245402. [Google Scholar] [CrossRef] [Green Version]
- Njoki, P.N.; Lim, I.S.; Mott, D.; Park, H.-Y.; Khan, B.; Mishra, S.; Sujakumar, R.; Luo, J.; Zhong, C.-J. Size Correlation of Optical and Spectroscopic Properties for Gold Nanoparticles. J. Phys. Chem. C 2007, 111, 14664–14669. [Google Scholar] [CrossRef]
- Liu, L.; Arenal, R.; Meira, D.M.; Corma, A. Generation of gold nanoclusters encapsulated in an MCM-22 zeolite for the aerobic oxidation of cyclohexane. Chem. Commun. 2019, 55, 1607–1610. [Google Scholar] [CrossRef] [Green Version]
- Turner, M.; Golovko, V.B.; Vaughan, O.P.H.; Abdulkin, P.; Berenguer-Murcia, A.; Tikhov, M.S.; Johnson, B.F.G.; Lambert, R.M. Selective oxidation with dioxygen by gold nanoparticle catalysts derived from 55-atoms clusters. Nature 2008, 454, 981–984. [Google Scholar] [CrossRef]
- Donoeva, B.J.; Ovoshchnikov, D.S.; Golovko, V.B. Establishing a Au Nanoparticle Size Effect in the Oxidation of Cyclohexene Using Gradually Changing Au Catalysts. ACS Catal. 2013, 3, 2986–2991. [Google Scholar] [CrossRef]
- Alshammari, H.; Miedziak, P.J.; Davis, T.H.; Knight, D.W.; Hutchings, J.G. Initiator-free hydrocarbon oxidation using supported gold nanoparticles. Catal. Sci. Technol. 2014, 4, 908–912. [Google Scholar] [CrossRef]
- Schünemann, S.; Dodekatos, G.; Tüysuz, H. Mesoporous Silica Supported Au and AuCu Nanoparticles for Surface Plasmon Driven Glycerol Oxidation. Chem. Mater. 2015, 27, 7743–7750. [Google Scholar] [CrossRef]










| Sample | Au (wt.%) | S (wt.%) | C (wt.%) | N (wt.%) | C/S (mol/mol) | S/Si (mol/mol) | TGA 170–400 °C (wt.% loss) | TGA 400–900 °C (wt.% loss) |
|---|---|---|---|---|---|---|---|---|
| SBA-SH | 0 | 1.33 | 3.11 | 0.03 | 6.3 | 0.027 | 4.0 | 1.9 |
| 3d-Au | 2.69 | 1.24 | 5.16 | 0.14 | 11.1 | 0.026 | 3.8 | 4.2 |
| 3d-Aux2 | 1.45 | 1.25 | 4.78 | 0.11 | 10.2 | 0.027 | 4.4 | 3.6 |
| 8d-Au | 0.31 | 1.25 | 5.31 | 0.13 | 11.4 | 0.027 | 4.3 | 3.6 |
| Sample | d-Spacing d100 (nm) | Unit Cell Parameter a0 (nm) | BET Surface Area (m2/g) | Total Pore Volume (cm3/g) | Micropore Volume (cm3/g) | Mesopore Volume (cm3/g) | Mesopore Size (nm) |
|---|---|---|---|---|---|---|---|
| SBA-15c | 9.275 | 10.7 | 742 | 0.79 | 0.09 | 0.70 | 8.7 |
| SBA-SH | 9.278 | 10.7 | 689 | 0.74 | 0.06 | 0.68 | 8.3 |
| 3d-Au | 9.297 | 10.7 | 587 | 0.63 | 0.05 | 0.58 | 8.1 |
| 3d-Aux2 | 9.304 | 10.7 | 606 | 0.66 | 0.05 | 0.61 | 8.1 |
| 8d-Au | 9.411 | 10.9 | 614 | 0.66 | 0.05 | 0.61 | 8.2 |
| Sample | BE (eV) | Surface Atomic Ratio | Bulk Atomic Ratio | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Si 2p | S 2p3/2 | S 2p1/2 | Au 4f7/2 | Au 4f5/2 | S/Si | Au/Si | Au/S | Sox/S a | S/Si | Au/Si | Au/S | |
| SBA-SH | 103.4 | 163.3 | 164.5 | - | - | 0.008 | - | - | 0 | 0.027 | 0 | 0 |
| 3d-Au | 103.5 | 163.3 | 164.5 | 84.6 | 88.2 | 0.007 | 0.007 | 1.11 | 0.58 | 0.026 | 0.009 | 0.356 |
| 168.5 | 169.7 | |||||||||||
| 8d-Au | 103.5 | 163.3 | 164.5 | 84.9 | 88.3 | 0.008 | 0.001 | 0.15 | 0.61 | 0.027 | 0.011 | 0.041 |
| 168.5 | 169.7 | |||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delgado, R.; Márquez-Álvarez, C.; Mayoral, Á.; de la Serna, R.; Agúndez, J.; Pérez-Pariente, J. Gold Clusters Immobilized by Post-Synthesis Methods on Thiol-Containing SBA-15 Mesoporous Materials for the Aerobic Oxidation of Cyclohexene: Influence of Light and Hydroperoxide. Chemistry 2023, 5, 526-543. https://doi.org/10.3390/chemistry5010038
Delgado R, Márquez-Álvarez C, Mayoral Á, de la Serna R, Agúndez J, Pérez-Pariente J. Gold Clusters Immobilized by Post-Synthesis Methods on Thiol-Containing SBA-15 Mesoporous Materials for the Aerobic Oxidation of Cyclohexene: Influence of Light and Hydroperoxide. Chemistry. 2023; 5(1):526-543. https://doi.org/10.3390/chemistry5010038
Chicago/Turabian StyleDelgado, Rafael, Carlos Márquez-Álvarez, Álvaro Mayoral, Ramón de la Serna, Javier Agúndez, and Joaquín Pérez-Pariente. 2023. "Gold Clusters Immobilized by Post-Synthesis Methods on Thiol-Containing SBA-15 Mesoporous Materials for the Aerobic Oxidation of Cyclohexene: Influence of Light and Hydroperoxide" Chemistry 5, no. 1: 526-543. https://doi.org/10.3390/chemistry5010038