Superspreading Surfactant on Hydrophobic Porous Substrates
Abstract
:1. Introduction
1.1. Stages of Spreading/Penetration over Porous Substrates
1.2. Partial Wetting
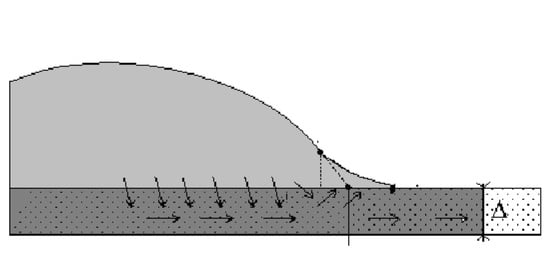
1.3. Complete Wetting Case
2. Materials and Methods
2.1. Materials
2.2. Contact Angle, Radius and Volume Measurements
3. Results and Discussion
3.1. Behavior of Water on PVDF Substrates
3.2. S 240 on Non-Porous Hydrophobic Film of PVDF
3.3. Behavior of Trisiloxane Surfactant Solutions S 240 on PVDF Membrane (Porous)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guan, J.H.; Wells, G.G.; Xu, B.; Mchale, G.; Wood, D.; Martin, J.; Stuart-cole, S. Evaporation of Sessile Droplets on Slippery Liquid-Infused Porous Surfaces (SLIPS). Langmuir 2015, 31, 11781–11789. [Google Scholar] [CrossRef]
- Starov, V.M.; Kostvintsev, S.R.; Sobolev, V.D.; Velarde, M.G.; Zhdanov, S.A. Spreading of liquid drops over dry porous layers: Complete wetting case. J. Colloid Interface Sci. 2002, 252, 397–408. [Google Scholar] [CrossRef]
- Calvert, P. Inkjet printing for materials and devices. Chem. Mater. 2001, 13, 3299–3305. [Google Scholar] [CrossRef]
- Genina, N.; Fors, D.; Vakili, H.; Ihalainen, P.; Pohjala, L.; Ehlers, H.; Kassamakov, I.; Haeggström, E.; Vuorela, P.; Peltonen, J.; et al. Tailoring controlled-release oral dosage forms by combining inkjet and flexographic printing techniques. Eur. J. Pharm. Sci. 2012, 47, 615–623. [Google Scholar] [CrossRef]
- Derby, B. Inkjet printing of functional and structural materials: Fluid property requirements, feature stability, and resolution. Annu. Rev. Mater. Res. 2010, 40, 395–414. [Google Scholar] [CrossRef]
- Kisi, O.; Khosravinia, P.; Heddam, S.; Karimi, B.; Karimi, N. Modeling wetting front redistribution of drip irrigation systems using a new machine learning method: Adaptive neuro-fuzzy system improved by hybrid particle swarm optimization—Gravity search algorithm. Agric. Water Manag. 2021, 256, 107067. [Google Scholar] [CrossRef]
- Wei, Q.; Xu, J.; Yang, S.; Qi, Z.; Wang, Y.; Liao, L. Partial wetting irrigation resulted in non-uniformly low nitrous oxide emissions from soil. Atmos. Environ. 2017, 161, 200–209. [Google Scholar] [CrossRef]
- Gambaryan-Roisman, T. Liquids on porous layers: Wetting, imbibition and transport processes. Curr. Opin. Colloid Interface Sci. 2014, 19, 320–335. [Google Scholar] [CrossRef]
- Li, X.; Fan, X. Pore wetting phenomena: Implications to enhanced oil recovery and geologic carbon storage. Energy Procedia 2014, 61, 2712–2715. [Google Scholar] [CrossRef]
- Cebeci, Y.; Sönmez, I. A study on the relationship between critical surface tension of wetting and oil agglomeration recovery of calcite. J. Colloid Interface Sci. 2004, 273, 300–305. [Google Scholar] [CrossRef]
- Sönmez, I.; Cebeci, Y. Investigation of relationship between critical surface tension of wetting and oil agglomeration recovery of barite. Colloids Surfaces A Physicochem. Eng. Asp. 2004, 234, 27–33. [Google Scholar] [CrossRef]
- Bhattacharjee, D.; Nazaripoor, H.; Soltannia, B.; Ismail, M.F.; Sadrzadeh, M. An experimental and numerical study of droplet spreading and imbibition on microporous membranes. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 615, 126191. [Google Scholar] [CrossRef]
- Kovalchuk, N.M.; Simmons, M.J.H. Surfactant-mediated wetting and spreading: Recent advances and applications. Curr. Opin. Colloid Interface Sci. 2021, 51, 101375. [Google Scholar] [CrossRef]
- Singh, S.K.; Khandekar, S.; Pratap, D.; Ramakrishna, S.A. Wetting dynamics and evaporation of sessile droplets on nano-porous alumina surfaces. Colloids Surfaces A Physicochem. Eng. Asp. 2013, 432, 71–81. [Google Scholar] [CrossRef]
- Marmur, A. Wetting on hydrophobic rough surfaces: To be heterogeneous or not to be? Langmuir 2003, 19, 8343–8348. [Google Scholar] [CrossRef]
- Alleborn, N.; Raszillier, H. Spreading and sorption of a droplet on a porous substrate. Chem. Eng. Sci. 2004, 59, 2071–2088. [Google Scholar] [CrossRef]
- Das, S.; Narayanam, C.; Roy, S.; Khanna, R. A model of wetting of partially wettable porous solids by thin liquid films. Chem. Eng. J. 2017, 320, 104–115. [Google Scholar] [CrossRef]
- Alleborn, N.; Raszillier, H. Spreading and sorption of droplets on layered porous substrates. J. Colloid Interface Sci. 2004, 280, 449–464. [Google Scholar] [CrossRef]
- Starov, V.; Velarde, M. Wetting and Spreading Dynamics, 2nd ed.; (Surfactantr Science Book 12); CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Sun, Y.-P.; Xin, Y.; Lyu, F.-T.; Dai, C.-L. Experimental study on the mechanism of adsorption-improved imbibition in oil-wet tight sandstone by a nonionic surfactant for enhanced oil recovery. Pet. Sci. 2021, 18, 1115–1126. [Google Scholar] [CrossRef]
- Ritacco, H.; Kurlat, D.H. Critical aggregation concentration in the PAMPS (10%)/DTAB system. Colloids Surf. A Physicochem. Eng. Asp. 2003, 218, 27–45. [Google Scholar] [CrossRef]
- Zhang, X.; Li, F.; Zhao, X. Treatment of surfactants with concentrations below critical micelle concentration by ultrafiltration: A mini-review. Water Cycle 2022, 3, 50–55. [Google Scholar] [CrossRef]
- Ivanova, N.; Starov, V.; Rubio, R.; Ritacco, H.; Hilal, N.; Johnson, D. Critical wetting concentrations of trisiloxane surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2010, 354, 143–148. [Google Scholar] [CrossRef]
- Theodorakis, P.E.; Müller, E.A.; Craster, R.V.; Matar, O.K. Superspreading: Mechanisms and molecular design. Langmuir 2015, 31, 2304–2309. [Google Scholar] [CrossRef] [PubMed]
- Venzmer, J. Superspreading—20 years of physicochemical research. Curr. Opin. Colloid Interface Sci. 2011, 16, 335–343. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tafireyi, W.; Littlewood, M.; Bandulasena, H.C.H.; Trybala, A.; Starov, V.M. Superspreading Surfactant on Hydrophobic Porous Substrates. Colloids Interfaces 2023, 7, 38. https://doi.org/10.3390/colloids7020038
Tafireyi W, Littlewood M, Bandulasena HCH, Trybala A, Starov VM. Superspreading Surfactant on Hydrophobic Porous Substrates. Colloids and Interfaces. 2023; 7(2):38. https://doi.org/10.3390/colloids7020038
Chicago/Turabian StyleTafireyi, Wellington, Max Littlewood, Himiyage Chaminda Hemaka Bandulasena, Anna Trybala, and Victor Mikhilovich Starov. 2023. "Superspreading Surfactant on Hydrophobic Porous Substrates" Colloids and Interfaces 7, no. 2: 38. https://doi.org/10.3390/colloids7020038