Effect of Surfactants/Gels on the Stability of Boron Particle Dispersion in Liquid Fuel
Abstract
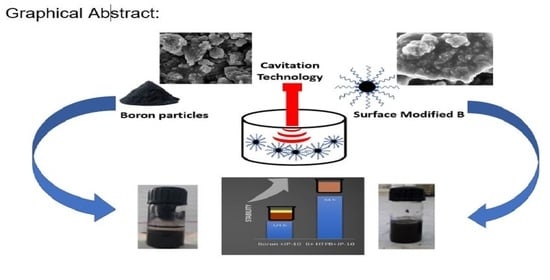
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methodology
2.3. Characterization
3. Results and Discussion
3.1. Morphological Studies
3.2. Effect of Ultrasound Irradiation on the Suspension of Boron Nanoparticles and JP-10
4. Characteristics, Thermal Behavior, and Stability of the Boron -JP-10 Suspension
4.1. Physical Properties of Boron-JP-10 Suspension
4.2. Thermal Characteristics of the Boron -JP-10 Suspension with Different Compositions
4.3. Stability Investigations of JP-10 Fuel Boron Particles through Visual Observation
4.4. Comparative Analysis of Boron-JP-10 Suspension
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hileman, J.I.; Stratton, R.W.; Donohoo, P.E. Energy content and alternative jet fuel viability. J. Propuls. Power 2010, 26, 1184–1196. [Google Scholar] [CrossRef]
- Cabrera, E.; de Sousa, J.M.M. Use of Sustainable Fuels in Aviation—A Review. Energies 2022, 15, 2440. [Google Scholar] [CrossRef]
- Yusaf, T.; Fernandes, L.; Abu Talib, A.R.; Altarazi, Y.S.; Alrefae, W.; Kadirgama, K.; Ramasamy, D.; Jayasuriya, A.; Brown, G.; Mamat, R.; et al. Sustainable aviation—Hydrogen is the future. Sustainability 2022, 14, 548. [Google Scholar] [CrossRef]
- Fortin, T.J.; Bruno, T.J. Heat capacity measurements of conventional aviation fuels. Fuel Process. Technol. 2022, 235, 107341. [Google Scholar] [CrossRef]
- Zhong, B.-J.; Zeng, Z.-M.; Zhang, H.-Z. An experimental and kinetic modeling study of JP-10 combustion. Fuel 2021, 312, 122900. [Google Scholar] [CrossRef]
- Shi, L.; Xu, P.; Wang, R.; Tang, W.; Ding, T.; Jiang, R.; Zhang, C. Experimental and kinetic study on JP-10/air autoignition and the effect of NO2 at high temperatures. Fuel 2023, 333, 126418. [Google Scholar] [CrossRef]
- Chen, A.; Guan, X.; Li, X.; Zhang, B.; Zhang, B.; Song, J. Preparation and Characterization of Metalized JP-10 Gel Propellants with Excellent Thixotropic Performance. Propellants Explos. Pyrotech. 2017, 42, 1007–1013. [Google Scholar] [CrossRef]
- Li, G.; Li, N.; Wang, X.; Sheng, X.; Li, S.; Wang, A.; Cong, Y.; Wang, X.; Zhang, T. Synthesis of diesel or jet fuel range cycloalkanes with 2-methylfuran and cyclopentanone from lignocellulose. Energy Fuels 2014, 28, 5112–5118. [Google Scholar] [CrossRef]
- Soudagar, M.E.M.; Nik-Ghazali, N.N.; Kalam, M.A.; Badruddin, I.A.; Banapurmath, N.R.; Akram, N. The effect of nano-additives in diesel-biodiesel fuel blends: A comprehensive review on stability, engine performance and emission characteristics. Energy Convers. Manag. 2018, 178, 146–177. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, L.; Zhang, X.; Han, P.; Xie, J.; Pan, L.; Zou, D.-R.; Liu, S.-H.; Zou, J.-J. Synthesis of high-density and low-freezing-point jet fuel using lignocellulose-derived isophorone and furanic aldehydes. Sustain. Energy Fuels 2018, 2, 1863–1869. [Google Scholar] [CrossRef]
- Chen, B.H.; Liu, J.Z.; Yao, F.; Li, H.P.; Zhou, J.H. Effect of oleic acid on the stability and rheology of nanoaluminium/JP-10 bi-phase system. Micro Nano Lett. 2017, 12, 675–679. [Google Scholar] [CrossRef]
- Gupta, S.K.; Prabhudeva, P.; Kumar, M.; Ojha, P.K.; Karmakar, S. Investigation on spray combustion characteristics of Boron-Loaded slurry fuel in a Swirl-Stabilized combustor. Fuel 2022, 323, 124316. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, X.; Zhang, J.; Zhu, Z.; Ren, X.; Yang, Y.; Lin, K.; Pang, A.; Shuai, Y. Encapsulated boron-based energetic spherical composites with improved reaction efficiency and combustion performance. Chem. Eng. J. 2022, 433, 134478. [Google Scholar] [CrossRef]
- Jin, Y.; Xu, X.; Wang, X.; Dou, S.; Yang, Q.; Pan, L. Propulsive and combustion behavior of hydrocarbon fuels containing boron nanoparticles in a liquid rocket combustor. Proc. Inst. Mech. Eng. Part G J. Aerosp. Eng. 2022, 236, 2580–2591. [Google Scholar] [CrossRef]
- Brotton, S.J.; Perera, S.D.; Misra, A.; Kleimeier, N.F.; Turner, A.M.; Kaiser, R.I.; Palenik, M.; Finn, M.T.; Epshteyn, A.; Sun, B.J.; et al. Combined spectroscopic and computational investigation on the oxidation of exo-tetrahydrodicyclopentadiene (jp-10; c10h16) doped with titanium–aluminum–boron reactive metal nanopowder. J. Phys. Chem. A 2021, 126, 125–144. [Google Scholar] [CrossRef] [PubMed]
- Xiu, E.; Zhi, X.; Zhang, Y.; Li, C.; Zou, J.J.; Zhang, X.; Wang, L. Jet fuel containing ligand-protecting energetic nanoparticles: A case study of boron in JP-10. Chem. Eng. Sci. 2015, 129, 9–13. [Google Scholar]
- Ferrão, I.A.S.; Mendes, M.A.A.; Moita, A.S.O.H.; Silva, A.R.R. The Addition of Particles to an Alternative Jet Fuel. Fuels 2022, 3, 184–206. [Google Scholar] [CrossRef]
- Shariatmadar, F.S.; Pakdehi, S.G. Effect of various surfactants on the stability time of kerosene–boron nanofluids. Micro Nano Lett. 2016, 11, 498–502. [Google Scholar] [CrossRef]
- Xue, K.; Cao, J.; Pan, L.; Zhang, X.; Zou, J.-J. Review on design, preparation and performance characterization of gelled fuels for advanced propulsion. Front. Chem. Sci. Eng. 2022, 16, 819–837. [Google Scholar] [CrossRef]
- Sandhya, M.; Ramasamy, D.; Sudhakar, K.; Kadirgama, K.; Harun, W. Ultrasonication an intensifying tool for preparation of stable nanofluids and study the time influence on distinct properties of graphene nanofluids—A systematic overview. Ultrason. Sonochem. 2021, 73, 105479. [Google Scholar] [CrossRef]
- Han, J.; Hu, S.; Liu, L.; He, P. High-Pressure Combustion Characteristics of Hydroxyl-Terminated Polybutadiene Propellants. J. Propuls. Power 2022, 39, 158–166. [Google Scholar] [CrossRef]
- Kawata, K.; Chung, H.L.; Itabashi, M. Mechanical characterization and high velocity ductility of HTPB propellant binder. In Composite Materials, 6th Japan US Conferences; CRC Press: Boca Raton, FL, USA, 2022; pp. 771–781. [Google Scholar]
- Amado, J.C.Q.; Ross, P.G.; Murakami, L.M.S.; Dutra, J.C.N. Properties of Hydroxyl-Terminal Polybutadiene (HTPB) and Its Use as a Liner and Binder for Composite Propellants: A Review of Recent Advances. Propellants Explos. Pyrotech. 2022, 47, e202100283. [Google Scholar]
- Xu, Y.; Li, D.; Zhou, S.; Shen, Z.; Li, Y. A Rate-Dependent Constitutive Model of HTPB Propellant Based on Parallel Rheological Framework. Propellants Explos. Pyrotech. 2022, 47, e202100334. [Google Scholar] [CrossRef]
- Thomas, J.C.; Petersen, E.L. HTPB Heat of Formation: Literature Survey, Group Additive Estimations, and Theoretical Effects. AIAA J. 2022, 60, 1269–1282. [Google Scholar] [CrossRef]
- Rang, S.; Jeong, J.; Bhosale, V.K.; Kwon, S. Reactivity of hypergolic hybrid solid fuel with industrial grade hydrogen peroxide. Fuel 2022, 330, 125543. [Google Scholar] [CrossRef]
- Meng, X.; Tian, H.; Zhu, H.; Wang, Z.; Yu, R.; Guo, Z.; Cai, G. Effects of aluminum and aluminum hydride additives on the performance of hybrid rocket motors based on 95% hydrogen peroxide. Aerosp. Sci. Technol. 2022, 130, 107914. [Google Scholar] [CrossRef]
- Smith, K.K.; Redeker, N.D.; Rios, J.C.; Mecklenburg, M.H.; Marcischak, J.C.; Guenthner, A.J.; Ghiassi, K.B. Surface modification and functionalization of boron nitride nanotubes via condensation with saturated and unsaturated alcohols for high performance polymer composites. ACS Appl. Nano Mater. 2019, 2, 4053–4060. [Google Scholar] [CrossRef]
- Sreedhara, S.S.; Joardar, J.; Ravula, V.; Tata, N.R. Preparation and characterization of nanoboron by cryo-milling. Adv. Powder Technol. 2020, 31, 3824–3832. [Google Scholar] [CrossRef]
- Yang, M.; Jin, H.; Sun, Z.; Gui, R. Experimental synthesis, functionalized modifications and potential applications of monoelemental zero-dimensional boron nanomaterials. J. Mater. Chem. A 2022, 10, 5111–5146. [Google Scholar] [CrossRef]
- Shariatmadar, F.S.; Pakdehi, S.G. Synthesis and Characterization of Aviation Turbine Kerosene Nanofuel Containing Boron Nanoparticles. Appl. Therm. Eng. 2017, 112, 1195–1204. [Google Scholar] [CrossRef]








| S. No. | Surfactant | Chemical Formula | Molecular Weight (g/mol) | Density (g/mL) |
|---|---|---|---|---|
| 1 | JP-10 fuel | C10H16 | 136 | 0.93 |
| 2 | HTPB | C10H15.4O0.07 | 136.5 | 0.902 |
| 3 | Triton X-100 | C18H28O5 | 324.41192 | 1.07 |
| 4 | Span 80 | C24H44O6 | 428.603 | 1.068 |
| 5 | Oleic acid | C18H34O2 | 282.47 | 0.895 |
| 6 | SDS | CH3(CH2)11SO4Na | 288.372 | 1.01 |
| Boron Particles @ 0.5 wt.% Sedimentation Time (h) at 27 °C | |||||||
|---|---|---|---|---|---|---|---|
| JP-10 Fuel (mL) | Sonication Time (min) | Surfactants (wt.%) | HTPB | Triton X-100 | SDS | Oleic Acid | Span 80 |
| 10 | 20 | 0.3 | 54 | 27 | 23 | 19 | 16 |
| 10 | 20 | 0.5 | 47 | 18 | 14 | 9 | 8 |
| 10 | 20 | 1 | 29 | 13 | 11 | 8 | 7 |
| 10 | 20 | 1.5 | 19 | 11 | 7 | 6 | 4 |
| 10 | 20 | 2 | 11 | 6 | 4 | 3 | 1.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dilipkumar, P.; Sonawane, S.H.; Srinath, S. Effect of Surfactants/Gels on the Stability of Boron Particle Dispersion in Liquid Fuel. Colloids Interfaces 2023, 7, 37. https://doi.org/10.3390/colloids7020037
Dilipkumar P, Sonawane SH, Srinath S. Effect of Surfactants/Gels on the Stability of Boron Particle Dispersion in Liquid Fuel. Colloids and Interfaces. 2023; 7(2):37. https://doi.org/10.3390/colloids7020037
Chicago/Turabian StyleDilipkumar, P., Shirish H. Sonawane, and S. Srinath. 2023. "Effect of Surfactants/Gels on the Stability of Boron Particle Dispersion in Liquid Fuel" Colloids and Interfaces 7, no. 2: 37. https://doi.org/10.3390/colloids7020037