Interplay of Interfacial and Rheological Properties on Drainage Reduction in CO2 Foam Stabilised by Surfactant/Nanoparticle Mixtures in Brine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedures
2.2.1. Preparation of Surfactant Solutions and NP Dispersions
2.2.2. Foam Characterisation
2.2.3. Surface Tension
2.2.4. Viscosity
2.2.5. Dynamic Light Scattering
3. Results
3.1. CO2 Foam Behaviour of Cationic, Anionic, and Zwitterionic Surfactants in Brine
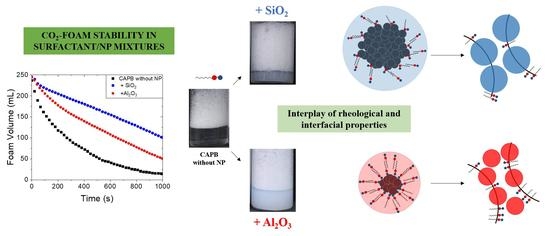
3.2. Effect of SiO2 and Al2O3 NPs on Foam Stability
3.3. Interfacial, Colloidal, and Rheological Properties of Surfactant/NP Systems: Implications for foam Destabilisation
3.4. Impact of Drainage Mechanism in the Presence of NPs on Foam Half-Life
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farajzadeh, R.; Andrianov, A.; Bruining, H.; Zitha, P.L.J. Comparative study of CO2 and N2 foams in porous media at low and high pressure-temperatures. Ind. Eng. Chem. Res. 2009, 48, 4542–4552. [Google Scholar] [CrossRef]
- Aarra, M.G.; Skauge, A.; Solbakken, J.; Ormehaug, P.A. Properties of N2- and CO2-foams as a function of pressure. J. Pet. Sci. Eng. 2014, 116, 72–80. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Han, Z.; He, C.; Feng, Q.; Wang, K.; Wang, Y.; Luo, N.; Dodbiba, G.; Wei, Y.; Otsuki, A.; et al. Long-term stability of different kinds of gas nanobubbles in deionized and salt water. Materials 2021, 14, 1808. [Google Scholar] [CrossRef] [PubMed]
- Roberto, P.G.; Arturo, B.T. Coalescence of Air Bubbles: Effect of the electrical double layer. Miner. Eng. 2020, 150, 106301. [Google Scholar] [CrossRef]
- Worthen, A.; Bryant, S.; Huh, C.; Johnston, K. Carbon dioxide-in-water foams stabilized with nanoparticles and surfactant acting in synergy. AlChE J. 2013, 59, 3490–3501. [Google Scholar] [CrossRef]
- Binks, B.P. Particles as surfactants—Similarities and differences. Curr. Opin. Colloid Interface Sci. 2002, 7, 21–41. [Google Scholar] [CrossRef]
- Guo, F.; Aryana, S. An experimental investigation of nanoparticle-stabilized CO2 foam used in enhanced oil recovery. Fuel 2016, 186, 430–442. [Google Scholar] [CrossRef]
- Emrani, A.S.; Nasr-El-Din, H.A. Stabilizing CO2 foam by use of nanoparticles. SPE J. 2017, 22, 494–504. [Google Scholar] [CrossRef]
- Binks, B.P.; Horozov, T.S. Aqueous foams stabilized solely by silica nanoparticles. Angew. Chem. Int. Ed. 2005, 44, 3722–3725. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Qiao, C.; Li, Z.; Wanambwa, S. Properties of carbon dioxide foam stabilized by hydrophilic nanoparticles and hexadecyltrimethylammonium bromide. Energy Fuels 2017, 31, 1478–1488. [Google Scholar] [CrossRef]
- Mohd, T.A.T.; Shukor, M.A.A.; Ghazali, N.A.; Alias, N.; Yahya, E.; Azizi, A.; Shahruddin, M.Z.; Ramleeh, N.A. Relationship between foamability and nanoparticle concentration of carbon dioxide (CO2) foam for enhanced oil recovery (EOR). Appl. Mech. Mater. 2014, 548–549, 67–71. [Google Scholar]
- Mohd, T.A.T.; Muhayyidin, A.H.M.; Ghazali, N.A.; Shahruddin, M.Z.; Alias, N.; Arina, S.; Ismail, S.N.; Ramlee, N.A. Carbon dioxide (CO2) foam stability dependence on nanoparticle concentration for enhanced oil recovery (EOR). Appl. Mech. Mater. 2014, 548–549, 1876–1880. [Google Scholar]
- Murshed, S.M.S.; Estellé, P. A state of the art review on viscosity of nanofluids. Renew. Sustain. Energy Rev. 2017, 76, 1134–1152. [Google Scholar] [CrossRef]
- Ab Rasid, S.A.; Mahmood, S.M.; Kechut, N.I.; Akbari, S. A review on parameters affecting nanoparticles stabilized foam performance based on recent analyses. J. Pet. Sci. Eng. 2022, 208, 109475. [Google Scholar] [CrossRef]
- Yekeen, N.; Idris, A.K.; Manan, M.A.; Samin, A.M. Experimental study of the influence of silica nanoparticles on the bulk stability of SDS-Foam in the presence of oil. J. Dispers. Sci. Technol. 2017, 38, 416–424. [Google Scholar] [CrossRef]
- Yekeen, N.; Padmanabhan, E.; Idris, A.K. Synergistic effects of nanoparticles and surfactants on n-decane-water interfacial tension and bulk foam stability at high temperature. J. Pet. Sci. Eng. 2019, 179, 814–830. [Google Scholar] [CrossRef]
- Zargartalebi, M.; Kharrat, R.; Barati, N. Enhancement of surfactant flooding performance by the use of silica nanoparticles. Fuel 2015, 143, 21–27. [Google Scholar] [CrossRef]
- Vatanparast, H.; Samiee, A.; Bahramian, A.; Javadi, A. Surface behavior of hydrophilic silica nanoparticle-SDS surfactant solutions: I. Effect of nanoparticle concentration on foamability and foam stability. Coll. Surfaces A Physicochem. Eng. Asp. 2017, 513, 430–441. [Google Scholar] [CrossRef]
- Zhao, J.; Torabi, F.; Yang, J. The synergistic role of silica nanoparticle and anionic surfactant on the static and dynamic CO2 foam stability for enhanced heavy oil recovery: An experimental study. Fuel 2021, 287, 119443. [Google Scholar] [CrossRef]
- Ma, L.; Zhu, M.; Liu, T. Effects of chain length of surfactants and their adsorption on nanoparticles on stability of CO2-in-water emulsions. Coll. Surfaces A Physicochem. Eng. Asp. 2022, 644, 128877. [Google Scholar] [CrossRef]
- Yekeen, N.; Manan, M.A.; Idris, A.K.; Padmanabhan, E.; Junin, R.; Samin, A.M.; Gbadamosi, A.O.; Oguamah, I. A comprehensive review of experimental studies of nanoparticles-stabilized foam for enhanced oil recovery. J. Pet. Sci. Eng. 2018, 164, 43–74. [Google Scholar] [CrossRef]
- Farhadi, H.; Riahi, S.; Ayatollahi, S.; Ahmadi, H. Experimental study of nanoparticle-surfactant-stabilized CO2 foam: Stability and mobility control. Chem. Eng. Res. Des. 2016, 111, 449–460. [Google Scholar] [CrossRef]
- Marčelja, S.; Lu, H.; Yuan, M.; Fang, B.; Wang, J.; Guo, Y.; Roberto, P.G.; Arturo, B.T.; Johnson, S.B.; Franks, G.V.; et al. Wormlike micelles in mixed amino acid-based anionic surfactant and zwitterionic surfactant systems. Langmuir 2018, 14, 8061–8074. [Google Scholar] [CrossRef] [Green Version]
- Lewis, J.A. Colloidal processing of ceramics. Adv. Appl. Ceram. 2004, 83, 2341–2359. [Google Scholar] [CrossRef]
- Drexler, S.; Silveira, T.M.G.; De Belli, G.; Couto, P. Experimental study of the effect of carbonated brine on wettability and oil displacement for EOR application in the Brazilian pre-salt reservoirs. Energy Sources Part A Recover. Util. Environ. Eff. 2021, 43, 3282–3296. [Google Scholar] [CrossRef]
- Rosen, M.J.; Kunjappu, J.T. Surfactants and Interfacial Phenomena; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012. [Google Scholar] [CrossRef]
- Wang, C.; Fang, H.; Gong, Q.; Xu, Z.; Liu, Z.; Zhang, L.; Zhang, L.; Zhao, S. Roles of catanionic surfactant mixtures on the stability of foams in the presence of oil. Energy Fuels 2016, 30, 6355–6364. [Google Scholar] [CrossRef]
- Petkova, B.; Tcholakova, S.; Chenkova, M.; Golemanov, K.; Denkov, N.; Thorley, D.; Stoyanov, S. Foamability of aqueous solutions: Role of surfactant type and concentration. Adv. Colloid Interface Sci. 2020, 276, 102084. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Yoon, R. Effects of surface forces and film elasticity on foam stability. Int. J. Miner. Process. 2008, 85, 101–110. [Google Scholar] [CrossRef]
- Hu, N.; Li, Y.; Wu, Z.; Lu, K.; Huang, D.; Liu, W. Foams stabilization by silica nanoparticle with cationic and anionic surfactants in column flotation: Effects of particle size. J. Taiwan Inst. Chem. Eng. 2018, 88, 62–69. [Google Scholar] [CrossRef]
- Wang, W.; Gu, B.; Liang, L. Effect of Surfactants on the formation, morphology, and surface property of synthesized SiO2 nanoparticles. J. Dispers. Sci. Technol. 2005, 25, 593–601. [Google Scholar] [CrossRef]
- Muhamad, M.S.; Salim, M.R.; Lau, W.-J. Surface modification of SiO2 nanoparticles and its impact on the properties of PES-based hollow fiber membrane. RSC Adv. 2015, 5, 58644–58654. [Google Scholar] [CrossRef]
- Yekeen, N.; Idris, A.K.; Manan, M.A.; Samin, A.M.; Risal, A.R.; Kun, T.X. Bulk and bubble-scale experimental studies of influence of nanoparticles on foam stability. Chin. J. Chem. Eng. 2017, 25, 347–357. [Google Scholar] [CrossRef]
- Rezaei, A.; Derikvand, Z.; Parsaei, R.; Imanivarnosfaderani, M. Surfactant-silica nanoparticle stabilized N2-foam flooding: A mechanistic study on the effect of surfactant type and temperature. J. Mol. Liq. 2021, 325, 115091. [Google Scholar] [CrossRef]
- Aladag, B.; Halelfadl, S.; Doner, N.; Maré, T.; Duret, S.; Estellé, P. Experimental investigations of the viscosity of nanofluids at low temperatures. Appl. Energy 2012, 97, 876–880. [Google Scholar] [CrossRef] [Green Version]
- Safouane, M.; Saint-Jalmes, A.; Bergeron, V.; Langevin, D. Viscosity effects in foam drainage: Newtonian and non-Newtonian foaming fluids. Eur. Phys. J. E 2006, 19, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Creatto, E.J.; Alvarenga, B.G.; de Moura, P.G.; Pérez-Gramatges, A. Viscosity-driven stabilization of CO2-in-brine foams using mixtures of cocamidopropyl hydroxysultaine and sodium dodecyl sulfate. J. Mol. Liq. 2021, 329, 115614. [Google Scholar] [CrossRef]
- Alvarenga, B.G.; Gonçalves, C.C.R.; Pérez-Gramatges, A. Stabilization of CO2-foams in brine by reducing drainage and coarsening using alkyldimethylamine oxides as surfactants. J. Mol. Liq. 2022, 347, 118370. [Google Scholar] [CrossRef]
- Wang, Z.; Ren, G.; Yang, J.; Xu, Z.; Sun, D. CO2-Responsive aqueous foams stabilized by pseudogemini surfactants. J. Colloid Interface Sci. 2019, 536, 381–388. [Google Scholar] [CrossRef]
- Da, C.; Jian, G.; Alzobaidi, S.; Yang, J.; Biswal, S.L.; Hirasaki, G.J.; Johnston, K.P. Design of CO2-in-water foam stabilized with switchable amine surfactants at high temperature in high-salinity brine and effect of oil. Energy Fuels 2018, 32, 12259–12267. [Google Scholar] [CrossRef]
- Xue, Z.; Worthen, A.; Qajar, A.; Robert, I.; Bryant, S.L.; Huh, C.; Prodanović, M.; Johnston, K.P. Viscosity and stability of ultra-high internal phase CO2-in-water foams stabilized with surfactants and nanoparticles with or without polyelectrolytes. J. Coll. Interface Sci. 2016, 461, 383–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravera, F.; Santini, E.; Loglio, G.; Ferrari, M.; Liggieri, L. Effect of nanoparticles on the interfacial properties of liquid/liquid and liquid/air surface layers. J. Phys. Chem. B 2006, 110, 19543–19551. [Google Scholar] [CrossRef] [PubMed]
- Romero, C.P.; Jeldres, R.I.; Quezada, G.R.; Concha, F.; Toledo, P.G. Zeta potential and viscosity of colloidal silica suspensions: Effect of seawater salts, PH, flocculant, and shear rate. Coll. Surf. A Physicochem. Eng. Asp. 2018, 538, 210–218. [Google Scholar] [CrossRef]
- Majumder, S.; Naskar, B.; Ghosh, S.; Lee, C.H.; Chang, C.H.; Moulik, S.P.; Panda, A.K. Synthesis and characterization of surfactant stabilized nanocolloidal dispersion of silver chloride in aqueous medium. Coll. Surf. A Physicochem. Eng. Asp. 2014, 443, 156–163. [Google Scholar] [CrossRef]
- Skoglund, S.; Blomberg, E.; Wallinder, I.O.; Grillo, I.; Pedersen, J.S.; Bergström, L.M. A novel explanation for the enhanced colloidal stability of silver nanoparticles in the presence of an oppositely charged surfactant. Phys. Chem. Chem. Phys. 2017, 19, 28037–28043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horozov, T.S. Foams and foam films stabilised by solid particles. Curr. Opin. Coll. Interface Sci. 2008, 13, 134–140. [Google Scholar] [CrossRef]
- Varade, D.; Carriere, D.; Arriaga, L.R.; Fameau, A.L.; Rio, E.; Langevin, D.; Drenckhan, W. On the origin of the stability of foams made from catanionic surfactant mixtures. Soft Matter 2011, 7, 6557–6570. [Google Scholar] [CrossRef]
- Saint-Jalmes, A. Physical chemistry in foam drainage and coarsening. Soft Matter 2006, 2, 836–849. [Google Scholar] [CrossRef]
- Wang, J.; Nguyen, A.V.; Farrokhpay, S. A critical review of the growth, drainage and collapse of foams. Adv. Coll. Interface Sci. 2016, 228, 55–70. [Google Scholar] [CrossRef] [Green Version]
- Koehler, S.A.; Hilgenfeldt, S.; Stone, H.A. Generalized view of foam drainage: Experiment and theory. Langmuir 2000, 16, 6327–6341. [Google Scholar] [CrossRef]
- Saint-Jalmes, A.; Langevin, D. Time evolution of aqueous foams: Drainage and coarsening. J. Phys. Condens. Matter 2002, 14, 9397–9412. [Google Scholar] [CrossRef]







| Chemical Name | Molecular Structure | CAS |
|---|---|---|
| Sodium dodecyl sulphate (SDS) |  | 151-21-3 |
| Dodecyl trimethyl ammonium bromide (DTAB) |  | 1119-94-4 |
| Cocamidopropyl betaine (CAPB) |  | 61789-40-0 |
| Silicon oxide (SiO2) NP |  | 7631-86-9 |
| Alumina oxide (Al2O3) NP |  | 1344-28-1 |
| Ion | Concentration (mg L−1) |
|---|---|
| Na+ | 22,016 |
| Ca2+ | 264 |
| Mg2+ | 302 |
| K+ | 786 |
| SO42− | 78 |
| Cl− | 35,873 |
| HCO3− | 72 |
| Surfactant | Foaming | Interfacial | |||||
|---|---|---|---|---|---|---|---|
| FF | t1/2 (s) | γmin (mN m−1) | Γ (mol m−2) | Am (Å2 molecule−1) | CMC (wt %) | pC20 | |
| SDS | 0.55 | 196 | 31.3 | 4.29 × 10−6 | 38.7 | 0.01 | 3.1 |
| DTAB | 0.51 | 39 | 38.1 | 4.44 × 10−6 | 37.4 | 0.05 | 2.0 |
| CAPB | 0.56 | 201 | 35.3 | 6.54 × 10−6 | 25.4 | 0.002 | 3.4 |
| DTAB | SDS | CAPB | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Surfactant/NP | Without NP | SiO2 | Al2O3 | Without NP | SiO2 | Al2O3 | Without NP | SiO2 | Al2O3 |
| CO2 foam t1/2 (s) | 39 | 250 | 50 | 196 | 265 | 737 | 201 | 846 | 511 |
| CO2 foam t1/2rel | 6.4 | 1.0 | 1.4 | 3.7 | 4.1 | 2.5 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Azevedo, B.R.S.; Alvarenga, B.G.; Percebom, A.M.; Pérez-Gramatges, A. Interplay of Interfacial and Rheological Properties on Drainage Reduction in CO2 Foam Stabilised by Surfactant/Nanoparticle Mixtures in Brine. Colloids Interfaces 2023, 7, 2. https://doi.org/10.3390/colloids7010002
de Azevedo BRS, Alvarenga BG, Percebom AM, Pérez-Gramatges A. Interplay of Interfacial and Rheological Properties on Drainage Reduction in CO2 Foam Stabilised by Surfactant/Nanoparticle Mixtures in Brine. Colloids and Interfaces. 2023; 7(1):2. https://doi.org/10.3390/colloids7010002
Chicago/Turabian Stylede Azevedo, Beatriz Ribeiro Souza, Bruno Giordano Alvarenga, Ana Maria Percebom, and Aurora Pérez-Gramatges. 2023. "Interplay of Interfacial and Rheological Properties on Drainage Reduction in CO2 Foam Stabilised by Surfactant/Nanoparticle Mixtures in Brine" Colloids and Interfaces 7, no. 1: 2. https://doi.org/10.3390/colloids7010002