Special Wettable Membranes for Oil/Water Separations: A Brief Overview of Properties, Types, and Recent Progress
Abstract
:1. Introduction
- I.
- More than 2 billion people live in water stress regions, and this is expected to increase dramatically in future;
- II.
- 1 billion people are suffering to get safe and clean drinking water;
- III.
- The usage of contaminated water is the reason for the death of 3.4 million people each year;
- IV.
- Millions of people collecting water from a distance of at least 6 km.
2. Fouling of the Membranes
- (a)
- Complete pore blocking;
- (b)
- Intermediate blocking;
- (c)
- Standard blocking;
- (d)
- Cake layer formation.
3. Contact Angle and Wettability Models
- (a)
- Hydrophilic: The water contact angle should be in the range of 10° < θ < 90°;
- (b)
- Hydrophobic: The water contact angle should be in the range of 90° < θ < 150°;
- (c)
- Superhydrophilic: The water contact angle should be in the range of 0° < θ < 10°;
- (d)
- Superhydrophobic: The water contact angle should be in the range of 150° < θ < 180°.
4. Brief Discussion of Polymers Used in Oil/Water Separation
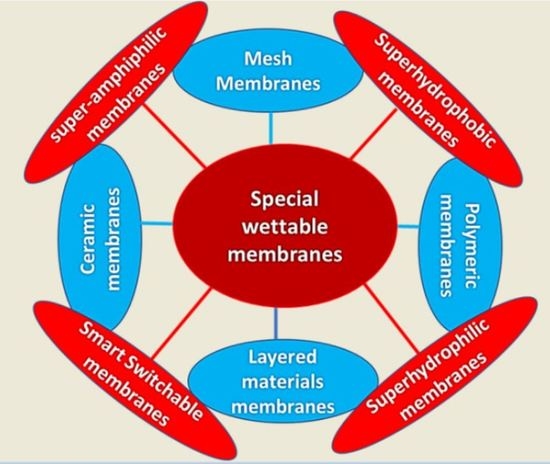
5. Types of Special Wettable Membranes
- (a)
- Superhydrophobic and superoleophilic materials;
- (b)
- Superhydrophilic and underwater superoleophobic materials.

5.1. Superhydrophobic and Superoleophilic Membranes
5.2. Superhydrophilic and Underwater Superoleophobic Membranes
6. Brief Discussion of the Mechanism of the Oil/Water Separation by Using Special Wettable Membranes
7. Challenges and Future Perspective
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, W.; Huang, J.; Li, S.; Ge, M.; Teng, L.; Chen, Z.; Lai, Y. Advanced Materials with Special Wettability toward Intelligent Oily Wastewater Remediation. ACS Appl. Mater. Interfaces 2021, 13, 67–87. [Google Scholar] [CrossRef]
- Rojas, S.; Horcajada, P. Metal-Organic Frameworks for the Removal of Emerging Organic Contaminants in Water. Chem. Rev. 2020, 120, 8378–8415. [Google Scholar] [CrossRef]
- He, C.; Liu, Z.; Wu, J.; Pan, X.; Fang, Z.; Li, J.; Bryan, B.A. Future Global Urban Water Scarcity and Potential Solutions. Nat. Commun. 2021, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tzanakakis, V.A.; Paranychianakis, N.V.; Angelakis, A.N. Water Supply and Water Scarcity. Water 2020, 12, 2347. [Google Scholar] [CrossRef]
- Baig, N.; Kammakakam, I. Special Wettable Azadirachta Indica Leaves like Microarchitecture Mesh Filtration Membrane Produced by Galvanic Replacement Reaction for Layered Oil/Water Separation. Chemosphere 2023, 313, 137544. [Google Scholar] [CrossRef]
- Baig, N.; Alowaid, A.M.; Abdulazeez, I.; Salhi, B.; Sajid, M.; Kammakakam, I. Designing of Nanotextured Inorganic-Organic Hybrid PVDF Membrane for Efficient Separation of the Oil-in-Water Emulsions. Chemosphere 2022, 308, 136531. [Google Scholar] [CrossRef]
- Li, L.; Xu, Z.; Sun, W.; Chen, J.; Dai, C.; Yan, B.; Zeng, H. Bio-Inspired Membrane with Adaptable Wettability for Smart Oil/Water Separation. J. Membrr. Sci. 2020, 598, 117661. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; Zeng, G.; Yang, Z.; Lin, Q.; Wang, Y.; Wang, X.; Pu, S. Two-Dimensional Na-Bentonite@MXene Composite Membrane with Switchable Wettability for Selective Oil/Water Separation. Sep. Purif. Technol 2023, 306, 122677. [Google Scholar] [CrossRef]
- Saien, J.; Shahrezaei, F. Organic Pollutants Removal from Petroleum Refinery Wastewater with Nanotitania Photocatalyst and UV Light Emission. Int. J. Photoenergy 2012, 2012. [Google Scholar] [CrossRef]
- Padaki, M.; Surya Murali, R.; Abdullah, M.S.; Misdan, N.; Moslehyani, A.; Kassim, M.A.; Hilal, N.; Ismail, A.F. Membrane Technology Enhancement in Oil–Water Separation. A Review. Desalination 2015, 357, 197–207. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Si, Y.; Zhang, S.; Yu, J. Tailoring Electrospun Nanofibrous Materials for Oil/Water Emulsion Separation. J. Text. Inst. 2021, 113, 2285–2298. [Google Scholar] [CrossRef]
- Baig, N.; Salhi, B.; Sajid, M.; Aljundi, I.H. Recent Progress in Microfiltration/Ultrafiltration Membranes for Separation of Oil and Water Emulsions. Chem. Rec. 2022, 22, e202100320. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liang, W.; Guo, Z.; Liu, W. Biomimetic Super-Lyophobic and Super-Lyophilic Materials Applied for Oil/Water Separation: A New Strategy beyond Nature. Chem. Soc. Rev. 2014, 44, 336–361. [Google Scholar] [CrossRef] [PubMed]
- Baig, N.; Saleh, T.A.; Baig, N.; Saleh, A. Superhydrophobic Polypropylene Functionalized with Nanoparticles for Efficient Fast Static and Dynamic Separation of Spilled Oil from Water. Glob. Chall. 2019, 3, 1800115. [Google Scholar] [CrossRef] [Green Version]
- Saleh, T.A.; Baig, N.; Alghunaimi, F.I.; Aljuryyed, N.W. A Flexible Biomimetic Superhydrophobic and Superoleophilic 3D Macroporous Polymer-Based Robust Network for the Efficient Separation of Oil-Contaminated Water. RSC Adv. 2020, 10, 5088–5097. [Google Scholar] [CrossRef] [Green Version]
- Baig, N.; Arshad, Z.; Ali, S.A. Synthesis of a Biomimetic Zwitterionic Pentapolymer to Fabricate High-Performance PVDF Membranes for Efficient Separation of Oil-in-Water Nano-Emulsions. Sci. Rep. 2022, 12, 1–15. [Google Scholar] [CrossRef]
- Cai, Y.; Shi, S.Q.; Fang, Z.; Li, J. Design, Development, and Outlook of Superwettability Membranes in Oil/Water Emulsions Separation. Adv. Mater. Interfaces 2021, 8, 2100799. [Google Scholar] [CrossRef]
- Zhu, H.; Guo, Z. Understanding the Separations of Oil/Water Mixtures from Immiscible to Emulsions on Super-Wettable Surfaces. J. Bionic Eng. 2016, 13, 1–29. [Google Scholar] [CrossRef]
- Lin, Y.M.; Rutledge, G.C. Separation of Oil-in-Water Emulsions Stabilized by Different Types of Surfactants Using Electrospun Fiber Membranes. J. Membrr. Sci. 2018, 563, 247–258. [Google Scholar] [CrossRef]
- Gogoi, M.; Goswami, R.; Borah, A.; Sarmah, H.; Rajguru, P.; Hazarika, S. Amide Functionalized DWCNT Nanocomposite Membranes for Chiral Separation of the Racemic DOPA. Sep. Purif. Technol. 2021, 279, 119704. [Google Scholar] [CrossRef]
- Borah, A.; Gogoi, M.; Goswami, R.; Sarmah, H.; Hazarika, K.K.; Hazarika, S. Thin Film Nanocomposite Membrane Incorporated with Clay-Ionic Liquid Framework for Enhancing Rejection of Epigallocatechin Gallate in Aqueous Media. J. Environ. Chem. Eng. 2022, 10, 107423. [Google Scholar] [CrossRef]
- Filippov, A.; Philippova, T. Control of Electrolyte Filtration through a Charged Porous Layer (Membrane) Using a Combination of Pressure Drop and an External Electric Field. Colloids Interfaces 2022, 6, 34. [Google Scholar] [CrossRef]
- Baig, N.; Matin, A.; Faizan, M.; Anand, D.; Ahmad, I.; Khan, S.A. Antifouling Low-Pressure Highly Permeable Single Step Produced Loose Nanofiltration Polysulfone Membrane for Efficient Erichrome Black T/Divalent Salts Fractionation. J. Environ. Chem. Eng. 2022, 10, 108166. [Google Scholar] [CrossRef]
- Dmitrieva, E.S.; Anokhina, T.S.; Novitsky, E.G.; Volkov, V.V.; Volkov, A.V.; Borisov, I.L. Polymeric Membranes for Oil-Water Separation: A Review. Polymers 2022, 14, 980. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zheng, Q.; Ding, L.; Yang, F.; Jin, W.; Tang, C.Y.; Dong, Y. Electro-Enhanced Separation of Microsized Oil-in-Water Emulsions via Metallic Membranes: Performance and Mechanistic Insights. Environ. Sci. Technol. 2022, 56, 4518–4530. [Google Scholar] [CrossRef]
- Gao, J.; Ma, S.; Xu, M.; Yuan, M.; Li, J.; Xue, J.; Wang, M. Photo-Fenton Superwettable NiFe2O4/TA/PVDF Composite Membrane for Organic Pollutant Degradation with Successively Oil-in-Water Separation. Chemosphere 2022, 286, 131705. [Google Scholar] [CrossRef]
- Dong, D.; Zhu, Y.; Fang, W.; Ji, M.; Wang, A.; Gao, S.; Lin, H.; Huang, R.; Jin, J. Double-Defense Design of Super-Anti-Fouling Membranes for Oil/Water Emulsion Separation. Adv. Funct. Mater. 2022, 32, 2113247. [Google Scholar] [CrossRef]
- Jiang, Q.; Wang, Y.; Xie, Y.; Zhou, M.; Gu, Q.; Zhong, Z.; Xing, W. Silicon Carbide Microfiltration Membranes for Oil-Water Separation: Pore Structure-Dependent Wettability Matters. Water Res. 2022, 216, 118270. [Google Scholar] [CrossRef]
- Ji, D.; Gao, Y.; Wang, W.; Feng, H.; Chen, K.; Xiao, C. Green Preparation of PVDF Hollow Fiber Membranes with Multiple Pore Structure via Melt Spinning Method for Oil/Water Separation. J. Environ. Chem. Eng. 2022, 10, 108337. [Google Scholar] [CrossRef]
- el Naggar, A.M.A.; Noor El-Din, M.R.; Mishrif, M.R.; Nassar, I.M. Highly Efficient Nano-Structured Polymer-Based Membrane/Sorbent for Oil Adsorption from O/W Emulsion Conducted of Petroleum Wastewater. J. Dispers. Sci. Technol. 2014, 36, 118–128. [Google Scholar] [CrossRef]
- Kulinich, S.; Kyzas, G.Z.; Fan, S.; Li, Y.; Wang, R.; Ma, W.; Shi, Y.; Fan, W.; Zhuo, K.; Xu, G. Intelligent Coatings with Controlled Wettability for Oil–Water Separation. Nanomaterials 2022, 12, 3120. [Google Scholar] [CrossRef]
- Wang, K.; Liu, X.; Dong, Y.; Zhang, S.; Li, J. A Biomimetic Janus Delignified Wood Membrane with Asymmetric Wettability Prepared by Thiol-Ol Chemistry for Unidirectional Water Transport and Selective Oil/Water Separation. Colloids Surf A Physicochem. Eng. Asp. 2022, 652, 129793. [Google Scholar] [CrossRef]
- Long, X.; Zhao, G.-Q.; Zheng, Y.; Hu, J.; Zuo, Y.; Zhang, J.; Jiao, F. Porous and Carboxyl Functionalized Titanium Carbide MXene Sheets for Fast Oil-in-Water Emulsion Separation. J. Membrr. Sci. 2022, 661, 120953. [Google Scholar] [CrossRef]
- Chen, C.; Weng, D.; Mahmood, A.; Chen, S.; Wang, J. Separation Mechanism and Construction of Surfaces with Special Wettability for Oil/Water Separation. ACS Appl. Mater. Interfaces 2019, 11, 11006–11027. [Google Scholar] [CrossRef] [PubMed]
- Baig, N.; Alghunaimi, F.I.; Saleh, T.A. Hydrophobic and Oleophilic Carbon Nanofiber Impregnated Styrofoam for Oil and Water Separation: A Green Technology. Chem. Eng. J. 2019, 360, 1613–1622. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, Y.; Liu, X.; Wang, D.; Li, J.; Jiang, L.; Jin, J. Salt-Induced Fabrication of Superhydrophilic and Underwater Superoleophobic PAA-g-PVDF Membranes for Effective Separation of Oil-in-Water Emulsions. Angew. Chem. Int. Ed. 2014, 53, 856–860. [Google Scholar] [CrossRef]
- He, S.; Zhan, Y.; Bai, Y.; Hu, J.; Li, Y.; Zhang, G.; Zhao, S. Gravity-Driven and High Flux Super-Hydrophobic/Super-Oleophilic Poly(Arylene Ether Nitrile) Nanofibrous Composite Membranes for Efficient Water-in-Oil Emulsions Separation in Harsh Environments. Compos. B Eng. 2019, 177, 107439. [Google Scholar] [CrossRef]
- Gao, J.; Wang, J.; Cai, M.; Xu, Q.; Zhang, J.; Cao, X.; Zhang, J.; Chen, Y. Advanced Superhydrophobic and Multifunctional Nanocellulose Aerogels for Oil/Water Separation: A Review. Carbohydr. Polym. 2023, 300, 120242. [Google Scholar] [CrossRef]
- Qu, M.; Ma, L.; Wang, J.; Shen, L.; Luo, Z.; Pang, Y.; He, J. Smart Materials with Special Wettability toward Oil/Water Separation and Recovery. ACS Symp. Ser. 2022, 1408, 77–106. [Google Scholar] [CrossRef]
- Su, Y.; Fan, T.; Cui, W.; Li, Y.; Ramakrishna, S.; Long, Y. Advanced Electrospun Nanofibrous Materials for Efficient Oil/Water Separation. Adv. Fiber Mater. 2022, 4, 938–958. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Z.; Liu, W. Special Superwetting Materials from Bioinspired to Intelligent Surface for On-Demand Oil/Water Separation: A Comprehensive Review. Small 2022, 18, 2204624. [Google Scholar] [CrossRef]
- Ge, M.; Cao, C.; Huang, J.; Zhang, X.; Tang, Y.; Zhou, X.; Zhang, K.; Chen, Z.; Lai, Y. Rational Design of Materials Interface at Nanoscale towards Intelligent Oil–Water Separation. Nanoscale Horiz 2018, 3, 235–260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Yang, X.; Wang, Y.; Qi, Y.; Zhang, Y.; Luo, J.; Cui, P.; Jiang, W. A Review on Oil/Water Emulsion Separation Membrane Material. J. Environ. Chem. Eng. 2022, 10, 107257. [Google Scholar] [CrossRef]
- Mao, X.; Wang, Y.; Yan, X.; Huang, Z.; Gao, Z.; Wang, Y.; Huang, L.; Kipper, M.J.; Tang, J. A Review of Superwetting Membranes and Nanofibers for Efficient Oil/Water Separation. J. Mater. Sci. 2022, 2022, 1–31. [Google Scholar] [CrossRef]
- Xiang, B.; Sun, Q.; Zhong, Q.; Mu, P.; Li, J. Current Research Situation and Future Prospect of Superwetting Smart Oil/Water Separation Materials. J. Mater. Chem. A Mater. 2022, 10, 20190–20217. [Google Scholar] [CrossRef]
- Baig, N. Two-Dimensional Nanomaterials: A Critical Review of Recent Progress, Properties, Applications, and Future Directions. Compos. Part A Appl. Sci. Manuf. 2023, 165, 107362. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Chen, M.; Liu, S.; Lai, B.; Tu, W. Strategies for the Construction of Special Wettability Metal Organic Framework Membranes: A Review. J. Water Process Eng. 2023, 51, 103374. [Google Scholar] [CrossRef]
- Sutar, R.S.; Latthe, S.S.; Gharge, N.B.; Gaikwad, P.P.; Jundle, A.R.; Ingole, S.S.; Ekunde, R.A.; Nagappan, S.; Park, K.H.; Bhosale, A.K.; et al. Facile Approach to Fabricate a High-Performance Superhydrophobic PS/OTS Modified SS Mesh for Oil-Water Separation. Colloids Surf. A Physicochem. Eng. Asp. 2023, 657, 130561. [Google Scholar] [CrossRef]
- Wang, D.; Gao, Y.; Gao, S.; Huang, H.; Min, F.; Li, Y.; Seeger, S.; Jin, J.; Chu, Z. Antifouling Superhydrophilic Porous Glass Membrane Based on Sulfobetaine Prepared by Thiol−ene Click Chemistry for High-Efficiency Oil/Water Separation. J. Membrr. Sci. 2023, 670, 121336. [Google Scholar] [CrossRef]
- Li, C.; Sun, W.; Lu, Z.; Ao, X.; Li, S. Ceramic Nanocomposite Membranes and Membrane Fouling: A Review. Water Res. 2020, 175, 115674. [Google Scholar] [CrossRef]
- Zulkefli, N.F.; Alias, N.H.; Jamaluddin, N.S.; Abdullah, N.; Manaf, S.F.A.; Othman, N.H.; Marpani, F.; Mat-Shayuti, M.S.; Kusworo, T.D. Recent Mitigation Strategies on Membrane Fouling for Oily Wastewater Treatment. Membranes 2022, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Alsawaftah, N.; Abuwatfa, W.; Darwish, N.; Husseini, G. A Comprehensive Review on Membrane Fouling: Mathematical Modelling, Prediction, Diagnosis, and Mitigation. Water 2021, 13, 1327. [Google Scholar] [CrossRef]
- Dickhout, J.M.; Moreno, J.; Biesheuvel, P.M.; Boels, L.; Lammertink, R.G.H.; de Vos, W.M. Produced Water Treatment by Membranes: A Review from a Colloidal Perspective. J. Colloid Interface Sci. 2017, 487, 523–534. [Google Scholar] [CrossRef]
- Tummons, E.; Han, Q.; Tanudjaja, H.J.; Hejase, C.A.; Chew, J.W.; Tarabara, V.V. Membrane Fouling by Emulsified Oil: A Review. Sep. Purif. Technol. 2020, 248, 116919. [Google Scholar] [CrossRef]
- Wang, F.; Tarabara, V.V. Pore Blocking Mechanisms during Early Stages of Membrane Fouling by Colloids. J. Colloid Interface Sci. 2008, 328, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Hoek, E.M.V.; Elimelech, M. Cake-Enhanced Concentration Polarization: A New Fouling Mechanism for Salt-Rejecting Membranes. Environ. Sci. Technol. 2003, 37, 5581–5588. [Google Scholar] [CrossRef]
- Ladewig, B.; Al-Shaeli, M.N.Z. Fouling in Membrane Bioreactors. In Fundamentals of Membrane Bioreactors; Springer Transactions in Civil and Environmental Engineering; Springer: Singapore, 2017; pp. 39–85. [Google Scholar] [CrossRef]
- Salhi, B.; Baig, N.; Abdulazeez, I.; Al-Ahmed, A.; Aljundi, I.H. High Flux Polyaniline-Coated Ceramic Membrane for Effective Separation of Emulsified Oil-in-Water. Ceram. Int. 2022, 48, 25246–25253. [Google Scholar] [CrossRef]
- Hussain, A.; Al-Yaari, M.; Hussain, A.; Al-Yaari, M. Development of Polymeric Membranes for Oil/Water Separation. Membranes 2021, 11, 42. [Google Scholar] [CrossRef]
- Salahi, A.; Mohammadi, T.; Abbasi, M.; Rekabdar, F. Chemical Cleaning of Ultrafiltration Membrane after Treatment of Oily Wastewater. Iran. J. Chem. Eng. 2010, 7, 17–28. [Google Scholar]
- Zhu, L.; Chen, M.; Dong, Y.; Tang, C.Y.; Huang, A.; Li, L. A Low-Cost Mullite-Titania Composite Ceramic Hollow Fiber Microfiltration Membrane for Highly Efficient Separation of Oil-in-Water Emulsion. Water Res. 2016, 90, 277–285. [Google Scholar] [CrossRef]
- Cai, Y.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. A Smart Membrane with Antifouling Capability and Switchable Oil Wettability for High-Efficiency Oil/Water Emulsions Separation. J. Membr. Sci. 2018, 555, 69–77. [Google Scholar] [CrossRef]
- Sun, L.; Guo, J.; Chen, H.; Zhang, D.; Shang, L.; Zhang, B.; Zhao, Y.; Sun, L.; Zhang, D.; Zhang, B.; et al. Tailoring Materials with Specific Wettability in Biomedical Engineering. Adv. Sci. 2021, 8, 2100126. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, Z. Recent Advances of Bioinspired Functional Materials with Specific Wettability: From Nature and beyond Nature. Nanoscale Horiz 2018, 4, 52–76. [Google Scholar] [CrossRef] [PubMed]
- Baig, N.; Alghunaimi, F.I.; Dossary, H.S.; Saleh, T.A. Superhydrophobic and Superoleophilic Carbon Nanofiber Grafted Polyurethane for Oil-Water Separation. Process Saf. Environ. Prot. 2019, 123, 327–334. [Google Scholar] [CrossRef]
- Kostal, E.; Stroj, S.; Kasemann, S.; Matylitsky, V.; Domke, M. Fabrication of Biomimetic Fog-Collecting Superhydrophilic-Superhydrophobic Surface Micropatterns Using Femtosecond Lasers. Langmuir 2018, 34, 2933–2941. [Google Scholar] [CrossRef]
- Saleh, T.A.; Baig, N. Efficient Chemical Etching Procedure for the Generation of Superhydrophobic Surfaces for Separation of Oil from Water. Prog. Org. Coat. 2019, 133, 27–32. [Google Scholar] [CrossRef]
- Zhao, T.; Jiang, L. Contact Angle Measurement of Natural Materials. Colloids Surf. B Biointerfaces 2018, 161, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Kim, D.; Kwak, K.; Nagata, Y.; Bonn, M.; Cho, M. Wettability of Graphene, Water Contact Angle, and Interfacial Water Structure. Chem 2022, 8, 1187–1200. [Google Scholar] [CrossRef]
- Parvate, S.; Dixit, P.; Chattopadhyay, S. Superhydrophobic Surfaces: Insights from Theory and Experiment. J. Phys. Chem. B 2020, 124, 1323–1360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, T. III. An Essay on the Cohesion of Fluids. Philos. Trans. R. Soc. Lond. 1805, 95, 65–87. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of Solid Surfaces to Wetting by Water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Baig, N.; Matin, A.; Khan, M.; Mansha, M.; Anand, D.; AlBalawi, N.; Nzila, A.M. Reverse Osmosis Membranes Functionalized with Polyglycidol Decorated Hyperbranched Copolymer Exhibits Superior Filtration Performance and Improved Fouling Resistance. J. Environ. Chem. Eng. 2022, 10, 108943. [Google Scholar] [CrossRef]
- Gogoi, M.; Goswami, R.; Borah, A.; Hazarika, S. In Situ Assembly of Functionalized Single-Walled Carbon Nanotube with Partially Reduced Graphene Oxide Nanocomposite Membrane for Chiral Separation of β-Substituted-α-Amino Acids. Sep. Purif. Technol. 2022, 283, 120201. [Google Scholar] [CrossRef]
- Zhang, G.; Yuan, S.; Cao, S.; Yan, G.; Wang, X.; Yang, J.; van der Bruggen, B. Functionalized Poly(Arylene Ether Sulfone) Containing Hydroxyl Units for the Fabrication of Durable, Superhydrophobic Oil/Water Separation Membranes. Nanoscale 2019, 11, 7166–7175. [Google Scholar] [CrossRef]
- Baruah, K.; Hazarika, S.; Borthakur, S.; Dutta, N.N. Preparation and Characterization of Polysulfone–Cyclodextrin Composite Nanofiltration Membrane: Solvent Effect. J. Appl. Polym. Sci. 2012, 125, 3888–3898. [Google Scholar] [CrossRef]
- Serbanescu, O.S.; Voicu, S.I.; Thakur, V.K. Polysulfone Functionalized Membranes: Properties and Challenges. Mater. Today Chem. 2020, 17, 100302. [Google Scholar] [CrossRef]
- Fane, A.G.; Wang, R.; Hu, M.X. Synthetic Membranes for Water Purification: Status and Future. Angew. Chem. Int. Ed. 2015, 54, 3368–3386. [Google Scholar] [CrossRef]
- Wang, H.H.; Jung, J.T.; Kim, J.F.; Kim, S.; Drioli, E.; Lee, Y.M. A Novel Green Solvent Alternative for Polymeric Membrane Preparation via Nonsolvent-Induced Phase Separation (NIPS). J. Membr. Sci. 2019, 574, 44–54. [Google Scholar] [CrossRef]
- Kumar, S.; Guria, C.; Mandal, A. Synthesis, Characterization and Performance Studies of Polysulfone/Bentonite Nanoparticles Mixed-Matrix Ultra-Filtration Membranes Using Oil Field Produced Water. Sep. Purif. Technol. 2015, 150, 145–158. [Google Scholar] [CrossRef]
- Jamshidi Gohari, R.; Halakoo, E.; Lau, W.J.; Kassim, M.A.; Matsuura, T.; Ismail, A.F. Novel Polyethersulfone (PES)/Hydrous Manganese Dioxide (HMO) Mixed Matrix Membranes with Improved Anti-Fouling Properties for Oily Wastewater Treatment Process. RSC Adv. 2014, 4, 17587–17596. [Google Scholar] [CrossRef]
- Nemade, P.R.; Ganjare, A.V.; Ramesh, K.; Rakte, D.M.; Vaishnavi, P.S.V.; Thapa, G. Low Fouling Sulphonated Carbon Soot-Polysulphone Membranes for Rapid Dehydration of Stabilized Oil-Water Emulsions. J. Water Process Eng. 2020, 38, 101590. [Google Scholar] [CrossRef]
- Liu, F.; Hashim, N.A.; Liu, Y.; Abed, M.R.M.; Li, K. Progress in the Production and Modification of PVDF Membranes. J. Membr. Sci. 2011, 375, 1–27. [Google Scholar] [CrossRef]
- Tang, F.; Wang, D.; Zhou, C.; Zeng, X.; Du, J.; Chen, L.; Zhou, W.; Lu, Z.; Tan, L.; Dong, L. Natural Polyphenol Chemistry Inspired Organic-Inorganic Composite Coating Decorated PVDF Membrane for Oil-in-Water Emulsions Separation. Mater. Res. Bull. 2020, 132, 110995. [Google Scholar] [CrossRef]
- Kang, G.D.; Cao, Y.M. Application and Modification of Poly(Vinylidene Fluoride) (PVDF) Membranes—A Review. J. Membr. Sci. 2014, 463, 145–165. [Google Scholar] [CrossRef]
- Zhao, Y.-H.; Zhu, B.-K.; Kong, L.; Xu, Y.-Y. Improving Hydrophilicity and Protein Resistance of Poly(Vinylidene Fluoride) Membranes by Blending with Amphiphilic Hyperbranched-Star Polymer. Langmuir 2007, 23, 5779–5786. [Google Scholar] [CrossRef] [PubMed]
- Yalcinkaya, F.; Siekierka, A.; Bryjak, M. Preparation of Fouling-Resistant Nanofibrous Composite Membranes for Separation of Oily Wastewater. Polymers 2017, 9, 679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, C.; Liu, G.; Qu, Z.; Wang, W.; Yu, D. GO/TiO2-Decorated Electrospun Polyvinylidene Fluoride Membrane Prepared Based on Metal-Polyphenol Coordination Network for Oil–Water Separation and Desalination. J. Mater. Sci. 2022, 57, 3452–3467. [Google Scholar] [CrossRef]
- Nayak, K.; Tripathi, B.P. Molecularly Grafted PVDF Membranes with In-Air Superamphiphilicity and Underwater Superoleophobicity for Oil/Water Separation. Sep. Purif. Technol. 2021, 259, 118068. [Google Scholar] [CrossRef]
- Nayak, K.; Kumar, A.; Tripathi, B.P. Molecular Grafting and Zwitterionization Based Antifouling and Underwater Superoleophobic PVDF Membranes for Oil/Water Separation. J. Membr. Sci. 2022, 643, 120038. [Google Scholar] [CrossRef]
- Boyraz, E.; Yalcinkaya, F.; Hruza, J.; Maryska, J. Surface-Modified Nanofibrous PVDF Membranes for Liquid Separation Technology. Materials 2019, 12, 2702. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Li, J.; Rao, L.; Lin, H.; Shen, L.; Xu, Y.; Chen, J.; Liao, B.Q. Inkjet Printing of Dopamine Followed by UV Light Irradiation to Modify Mussel-Inspired PVDF Membrane for Efficient Oil-Water Separation. J. Membr. Sci. 2021, 619, 118790. [Google Scholar] [CrossRef]
- Gao, J.; Wang, J.; Xu, Q.; Wu, S.; Chen, Y. Regenerated Cellulose Strongly Adhered by a Supramolecular Adhesive onto the PVDF Membrane for a Highly Efficient Oil/Water Separation. Green Chem. 2021, 23, 5633–5646. [Google Scholar] [CrossRef]
- Deng, W.; Fan, T.; Li, Y. In Situ Biomineralization-Constructed Superhydrophilic and Underwater Superoleophobic PVDF-TiO2 Membranes for Superior Antifouling Separation of Oil-in-Water Emulsions. J. Membr. Sci. 2021, 622, 119030. [Google Scholar] [CrossRef]
- Lin, Z.; Cao, N.; Li, C.; Sun, R.; Li, W.; Chen, L.; Sun, Y.; Zhang, H.; Pang, J.; Jiang, Z. Micro-Nanostructure Tuning of PEEK Porous Membrane Surface Based on PANI in-Situ Growth for Antifouling Ultrafiltration Membranes. J. Membr. Sci. 2022, 663, 121058. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Si, Y.; Yu, J.; Ding, B. Rational Design of Electrospun Nanofibrous Materials for Oil/Water Emulsion Separation. Mater. Chem. Front. 2021, 5, 97–128. [Google Scholar] [CrossRef]
- Jepsen, K.L.; Bram, M.V.; Pedersen, S.; Yang, Z. Membrane Fouling for Produced Water Treatment: A Review Study From a Process Control Perspective. Water 2018, 10, 847. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Si, Y.; Zhu, H.; Jiang, T.; Guo, Z. A Study on the Fabrication of Porous PVDF Membranes by In-Situ Elimination and Their Applications in Separating Oil/Water Mixtures and Nano-Emulsions. J. Membr. Sci. 2016, 520, 760–768. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, H.; Shao, Y.; Zhang, H.; Zhu, J. Recent Progresses of Superhydrophobic Coatings in Different Application Fields: An Overview. Coatings 2021, 11, 116. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, N.; Cao, Y.; Lin, X.; Liu, Y.; Feng, L. Superwetting Porous Materials for Wastewater Treatment: From Immiscible Oil/Water Mixture to Emulsion Separation. Adv. Mater. Interfaces 2017, 4, 1600029. [Google Scholar] [CrossRef]
- Nyankson, E.; Agbe, H.; Takyi, G.K.S.; Bensah, Y.D.; Sarkar, D.K. Recent Advances in Nanostructured Superhydrophobic Surfaces: Fabrication and Long-Term Durability Challenges. Curr. Opin. Chem. Eng. 2022, 36, 100790. [Google Scholar] [CrossRef]
- Zaman Khan, M.; Militky, J.; Petru, M.; Tomková, B.; Ali, A.; Tören, E.; Perveen, S. Recent Advances in Superhydrophobic Surfaces for Practical Applications: A Review. Eur. Polym. J. 2022, 178, 111481. [Google Scholar] [CrossRef]
- Baig, N.; Kammakakam, I. Removal of Oily Contaminants from Water by Using the Hydrophobic Ag Nanoparticles Incorporated Dopamine Modified Cellulose Foam. Polymers 2021, 13, 3163. [Google Scholar] [CrossRef]
- Teisala, H.; Tuominen, M.; Kuusipalo, J. Superhydrophobic Coatings on Cellulose-Based Materials: Fabrication, Properties, and Applications. Adv. Mater. Interfaces 2014, 1, 1300026. [Google Scholar] [CrossRef]
- Chauhan, K.v.; Desai, M.K.; Patel, A.C. Recent Progress in the Development and Anti-Icing Applications of Superhydrophobic Coatings. Mater. Today Proc. 2022, 62, 3922–3928. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Zhang, Y.Q.; Gao, C.; An, Q.D.; Xiao, Z.Y.; Zhai, S.R. Superhydrophobic Aerogel Membrane with Integrated Functions of Biopolymers for Efficient Oil/Water Separation. Sep. Purif. Technol. 2022, 282, 120138. [Google Scholar] [CrossRef]
- Yang, J.; He, T.; Li, X.; Wang, R.; Wang, S.; Zhao, Y.; Wang, H. Rapid Dipping Preparation of Superhydrophobic TiO2 Cotton Fabric for Multifunctional Highly Efficient Oil-Water Separation and Photocatalytic Degradation. Colloids Surf. A Physicochem. Eng. Asp. 2023, 657, 130590. [Google Scholar] [CrossRef]
- Duman, O.; Uğurlu, H.; Diker, C.Ö.; Tunç, S. Fabrication of Highly Hydrophobic or Superhydrophobic Electrospun PVA and Agar/PVA Membrane Materials for Efficient and Selective Oil/Water Separation. J. Environ. Chem. Eng. 2022, 10, 107405. [Google Scholar] [CrossRef]
- Zhao, S.; Tie, L.; Guo, Z.; Li, J. Water Deteriorates Lubricating Oils: Removal of Water in Lubricating Oils Using a Robust Superhydrophobic Membrane. Nanoscale 2020, 12, 11703–11710. [Google Scholar] [CrossRef]
- Pan, Z.; Cao, S.; Li, J.; Du, Z.; Cheng, F. Anti-Fouling TiO2 Nanowires Membrane for Oil/Water Separation: Synergetic Effects of Wettability and Pore Size. J. Membr. Sci. 2019, 572, 596–606. [Google Scholar] [CrossRef]
- Liu, N.; Yang, Z.; Sun, Y.; Shan, L.; Li, H.; Wang, Z. Slippery Mechanism for Enhancing Separation and Anti-Fouling of the Superhydrophobic Membrane in a Water-in-Oil Emulsion: Evaluating Water Adhesion of the Membrane Surface. Langmuir 2022, 38, 8312–8323. [Google Scholar] [CrossRef]
- Saleh, T.A.; Baig, N.; Othman, H.A.; al Harith, A.M. Removal of Alkanes by Novel Grassy Cabbage Microbuds Prepared by an Electrochemical Method. Chem. Eng. J. 2021, 407, 126216. [Google Scholar] [CrossRef]
- Lin, J.; Lin, F.; Liu, R.; Li, P.; Fang, S.; Ye, W.; Zhao, S. Scalable Fabrication of Robust Superhydrophobic Membranes by One-Step Spray-Coating for Gravitational Water-in-Oil Emulsion Separation. Sep. Purif. Technol. 2020, 231, 115898. [Google Scholar] [CrossRef]
- Baig, N.; Saleh, T.A. A Facile Development of Superhydrophobic and Superoleophilic Micro-Textured Functionalized Mesh Membrane for Fast and Efficient Separation of Oil from Water. J. Environ. Chem. Eng. 2021, 9, 105825. [Google Scholar] [CrossRef]
- Huang, F.; Li, Q.; Ji, G.; Tu, J.; Ding, N.; Qu, Q.; Liu, G. Oil/Water Separation Using a Lauric Acid-Modified, Superhydrophobic Cellulose Composite Membrane. Mater. Chem. Phys. 2021, 266, 124493. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, H.; Wang, E.; Zhang, X.; Yuan, R.; Zhu, Y. Superhydrophobic Poly(Vinylidene Fluoride) Membranes with Controllable Structure and Tunable Wettability Prepared by One-Step Electrospinning. Polymer 2016, 82, 105–113. [Google Scholar] [CrossRef]
- Ma, W.; Ding, Y.; Zhang, M.; Gao, S.; Li, Y.; Huang, C.; Fu, G. Nature-Inspired Chemistry toward Hierarchical Superhydrophobic, Antibacterial and Biocompatible Nanofibrous Membranes for Effective UV-Shielding, Self-Cleaning and Oil-Water Separation. J. Hazard. Mater. 2020, 384, 121476. [Google Scholar] [CrossRef]
- Li, D.; Gou, X.; Wu, D.; Guo, Z. A Robust and Stretchable Superhydrophobic PDMS/PVDF@KNFs Membrane for Oil/Water Separation and Flame Retardancy. Nanoscale 2018, 10, 6695–6703. [Google Scholar] [CrossRef]
- Fan, T.; Miao, J.; Li, Z.; Cheng, B. Bio-Inspired Robust Superhydrophobic-Superoleophilic Polyphenylene Sulfide Membrane for Efficient Oil/Water Separation under Highly Acidic or Alkaline Conditions. J. Hazard. Mater. 2019, 373, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Mayoussi, F.; Doeven, E.H.; Kick, A.; Goralczyk, A.; Thomann, Y.; Risch, P.; Guijt, R.M.; Kotz, F.; Helmer, D.; Rapp, B.E. Facile Fabrication of Micro-/Nanostructured, Superhydrophobic Membranes with Adjustable Porosity by 3D Printing. J. Mater. Chem. A Mater. 2021, 9, 21379–21386. [Google Scholar] [CrossRef]
- Tsai, Y.T.; Maggay, I.V.; Venault, A.; Lin, Y.F. Fluorine-Free and Hydrophobic/Oleophilic PMMA/PDMS Electrospun Nanofibrous Membranes for Gravity-Driven Removal of Water from Oil-Rich Emulsions. Sep. Purif. Technol. 2021, 279, 119720. [Google Scholar] [CrossRef]
- Su, Y.; Fan, T.; Bai, H.; Guan, H.; Ning, X.; Yu, M.; Long, Y. Bioinspired Superhydrophobic and Superlipophilic Nanofiber Membrane with Pine Needle-like Structure for Efficient Gravity-Driven Oil/Water Separation. Sep. Purif. Technol. 2021, 274, 119098. [Google Scholar] [CrossRef]
- Velayi, E.; Norouzbeigi, R. Selective Superantiwetting/Superwetting Fluorine-Free Nanostructured ZnO/CuO Mesh Membrane for Efficient Separation of Oil/Water Mixture: Oxygen Vacancy-Dependent Wetting Stability Studies. Surf. Coat. Technol. 2022, 430, 127992. [Google Scholar] [CrossRef]
- Wang, F.P.; Zhao, X.J.; Wahid, F.; Zhao, X.Q.; Qin, X.T.; Bai, H.; Xie, Y.Y.; Zhong, C.; Jia, S.R. Sustainable, Superhydrophobic Membranes Based on Bacterial Cellulose for Gravity-Driven Oil/Water Separation. Carbohydr. Polym. 2021, 253, 117220. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, T.C.; Wei, B.; Chen, S.; Liang, Y.; Yuan, S. Durable CNTs Reinforced Porous Electrospun Superhydrophobic Membrane for Efficient Gravity Driven Oil/Water Separation. Colloids Surf. A Physicochem. Eng. Asp. 2021, 608, 125342. [Google Scholar] [CrossRef]
- Sun, X.; Bai, L.; Li, J.; Huang, L.; Sun, H.; Gao, X. Robust Preparation of Flexibly Super-Hydrophobic Carbon Fiber Membrane by Electrospinning for Efficient Oil-Water Separation in Harsh Environments. Carbon 2021, 182, 11–22. [Google Scholar] [CrossRef]
- Kim, D.S.; Kang, J.; Jung, J.Y.; Hwang, M.; Seo, S.; Kim, J.H. Facile Fabrication of Superhydrophobic Polymer Membranes with Hierarchical Structure for Efficient Oil/Water Separation. Fibers Polym. 2022, 2022, 1–8. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, J.; Li, Y.; Zhang, X.; An, A.K.; Wang, Z. Superhydrophobic and Superoleophilic PH-CNT Membrane for Emulsified Oil-Water Separation. Desalination 2022, 526, 115536. [Google Scholar] [CrossRef]
- PV, V.M.; Kudapa, V.K. Recent Developments in Usage of Fluorine-Free Nano Structured Materials in Oil-Water Separation: A Review. Surf. Interfaces 2021, 27, 101455. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, M.; Liu, Z.; Kang, M.; Huang, C.; Fu, G. Fabrication of Highly Durable and Robust Superhydrophobic-Superoleophilic Nanofibrous Membranes Based on a Fluorine-Free System for Efficient Oil/Water Separation. J. Membr. Sci. 2019, 570–571, 303–313. [Google Scholar] [CrossRef]
- Huang, G.; Huo, L.; Jin, Y.; Yuan, S.; Zhao, R.; Zhao, J.; Li, Z.; Li, Y. Fluorine-Free Superhydrophobic PET Fabric with High Oil Flux for Oil–Water Separation. Prog. Org. Coat. 2022, 163, 106671. [Google Scholar] [CrossRef]
- Barthwal, S.; Lim, S.H. A Durable, Fluorine-Free, and Repairable Superhydrophobic Aluminum Surface with Hierarchical Micro/Nanostructures and Its Application for Continuous Oil-Water Separation. J. Membr. Sci. 2021, 618, 118716. [Google Scholar] [CrossRef]
- Ezugbe, E.O.; Rathilal, S. Membrane Technologies in Wastewater Treatment: A Review. Membranes 2020, 10. [Google Scholar] [CrossRef]
- Eang, C.; Nim, B.; Sreearunothai, P.; Petchsuk, A.; Opaprakasit, P. Chemical Upcycling of Polylactide (PLA) and Its Use in Fabricating PLA-Based Super-Hydrophobic and Oleophilic Electrospun Nanofibers for Oil Absorption and Oil/Water Separation. New J. Chem. 2022, 46, 14933–14943. [Google Scholar] [CrossRef]
- Zhao, J.; Deng, Y.; Dai, M.; Wu, Y.; Ali, I.; Peng, C. Preparation of Super-Hydrophobic/Super-Oleophilic Quartz Sand Filter for the Application in Oil-Water Separation. J. Water Process Eng. 2022, 46, 102561. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, Z.; Zhang, F.; Liu, X.; Jin, J.; Jiang, L. Superhydrophobic and Superoleophilic PVDF Membranes for Effective Separation of Water-in-Oil Emulsions with High Flux. Adv. Mater. 2013, 25, 2071–2076. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Wang, S.; Lin, L.; Chen, L.; Liu, M.; Feng, L.; Jiang, L. A Novel Superhydrophilic and Underwater Superoleophobic Hydrogel-Coated Mesh for Oil/Water Separation. Adv. Mater. 2011, 23, 4270–4273. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhan, Y.; Sun, A.; Feng, Q.; Yang, W.; Dong, H.; Chen, Y.; Zhang, Y. Anchoring the TiO2@crumpled Graphene Oxide Core–Shell Sphere onto Electrospun Polymer Fibrous Membrane for the Fast Separation of Multi-Component Pollutant-Oil–Water Emulsion. Sep. Purif. Technol. 2022, 298, 121605. [Google Scholar] [CrossRef]
- Qi, G.; Guo, K.; Yang, J.; Wang, Y.; Wang, Z.; Yuan, Z. A Stable Underwater Superoleophobic Membrane Constructed by CuO Oriented Rods and PAA Water-Adsorbent Resin for Fast and High Efficient Oil–Water Separation. Sep. Purif. Technol. 2022, 294, 121175. [Google Scholar] [CrossRef]
- Zhou, J.; Li, X.; Hou, T.; Zhang, X.; Yang, B. Biodegradable, Biomimetic, and Nanonet-Engineered Membranes Enable High-Flux and Highly-Efficient Oil/Water Separation. J. Hazard. Mater. 2022, 434, 128858. [Google Scholar] [CrossRef]
- Jin, Y.; Huang, L.; Zheng, K.; Zhou, S. Blending Electrostatic Spinning Fabrication of Superhydrophilic/Underwater Superoleophobic Polysulfonamide/Polyvinylpyrrolidone Nanofibrous Membranes for Efficient Oil-Water Emulsion Separation. Langmuir 2022, 38, 8241–8251. [Google Scholar] [CrossRef]
- Xie, W.; Chen, M.; Wei, S.; Huang, Z.; Li, Z. Lignin Nanoparticles-Intercalated Reduced Graphene Oxide/Glass Fiber Composite Membranes for Highly Efficient Oil-in-Water Emulsions Separation in Harsh Environment. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129190. [Google Scholar] [CrossRef]
- Shijie, F.; Jiefeng, Z.; Pengyu, Z.; Yunling, G.A.O.; Junxian, Y. Superhydrophilic/Underwater Superoleophobic Oil-in-Water Emulsion Separation Membrane Modified by the Co-Deposition of Polydopamine and Chitosan-Tripolyphosphate Nanoparticles. J. Environ. Chem. Eng. 2022, 10, 107407. [Google Scholar] [CrossRef]
- Zuo, Y.; Long, X.; Zheng, Y.; Zhang, J.; Wang, L.; Hu, J.; Jiao, F. Gelatin-Tannic Acid Coating for High Flux Oil-Water Separation. J. Environ. Chem. Eng. 2022, 10, 107992. [Google Scholar] [CrossRef]
- Cao, M.; Xiao, F.; Yang, Z.; Chen, Y.; Lin, L. Purification of Oil-Containing Emulsified Wastewater via PAN Nanofiber Membrane Loading PVP-UiO-66-NH2. Sep. Purif. Technol. 2022, 297, 121514. [Google Scholar] [CrossRef]
- Xu, Z.; Li, L.; Liu, J.; Dai, C.; Sun, W.; Chen, J.; Zhu, Z.; Zhao, M.; Zeng, H. Mussel-Inspired Superhydrophilic Membrane Constructed on a Hydrophilic Polymer Network for Highly Efficient Oil/Water Separation. J. Colloid Interface Sci. 2022, 608, 702–710. [Google Scholar] [CrossRef]
- Gao, Y.; Hao, W.; Xu, G.; Wang, C.; Gu, X.; Zhao, P. Enhancement of Super-Hydrophilic/Underwater Super-Oleophobic Performance of Ceramic Membrane with TiO2 Nanowire Array Prepared via Low Temperature Oxidation. Ceram. Int. 2022, 48, 9426–9433. [Google Scholar] [CrossRef]
- Feng, Q.; Zhan, Y.; Yang, W.; Sun, A.; Dong, H.; Chiao, Y.H.; Liu, Y.; Chen, X.; Chen, Y. Bi-Functional Super-Hydrophilic/Underwater Super-Oleophobic 2D Lamellar Ti3C2Tx MXene/Poly (Arylene Ether Nitrile) Fibrous Composite Membrane for the Fast Purification of Emulsified Oil and Photodegradation of Hazardous Organics. J. Colloid Interface Sci. 2022, 612, 156–170. [Google Scholar] [CrossRef]
- Ahmed, F.U.; Upadhaya, D.; Dhar Purkayastha, D.; Krishna, M.G. Stable Hydrophilic and Underwater Superoleophobic ZnO Nanorod Decorated Nanofibrous Membrane and Its Application in Wastewater Treatment. J. Membr. Sci. 2022, 659, 120803. [Google Scholar] [CrossRef]
- Kwon, G.; Post, E.; Tuteja, A. Membranes with Selective Wettability for the Separation of Oil–Water Mixtures. MRS Commun. 2015, 5, 475–494. [Google Scholar] [CrossRef]
- You, H.; Shangkum, G.Y.; Chammingkwan, P.; Taniike, T. Surface Wettability Switching of a Zeolitic Imidazolate Framework-deposited Membrane for Selective Efficient Oil/Water Emulsion Separation. Colloids Surf. A Physicochem. Eng. Asp. 2021, 614, 126204. [Google Scholar] [CrossRef]
- Solomon, B.R.; Hyder, M.N.; Varanasi, K.K. Separating Oil-Water Nanoemulsions Using Flux-Enhanced Hierarchical Membranes. Sci. Rep. 2014, 4, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Amoudi, A.S. Factors Affecting Natural Organic Matter (NOM) and Scaling Fouling in NF Membranes: A Review. Desalination 2010, 259, 1–10. [Google Scholar] [CrossRef]
- Luo, X.; He, Z.; Gong, H.; He, L. Recent Advances in Oil-Water Separation Materials with Special Wettability Modified by Graphene and Its Derivatives: A Review. Chem. Eng. Process.-Process Intensif. 2022, 170, 108678. [Google Scholar] [CrossRef]
- Wei, Y.; Qi, H.; Gong, X.; Zhao, S. Specially Wettable Membranes for Oil–Water Separation. Adv. Mater. Interfaces 2018, 5, 1800576. [Google Scholar] [CrossRef]
- Ma, Q.; Cheng, H.; Fane, A.G.; Wang, R.; Zhang, H. Recent Development of Advanced Materials with Special Wettability for Selective Oil/Water Separation. Small 2016, 12, 2186–2202. [Google Scholar] [CrossRef]
- Pulido, B.A.; Habboub, O.S.; Aristizabal, S.L.; Szekely, G.; Nunes, S.P. Recycled Poly(Ethylene Terephthalate) for High Temperature Solvent Resistant Membranes. ACS Appl. Polym. Mater. 2019, 1, 2379–2387. [Google Scholar] [CrossRef] [Green Version]
- Bhuyan, C.; Konwar, A.; Bora, P.; Rajguru, P.; Hazarika, S. Cellulose Nanofiber-Poly(Ethylene Terephthalate) Nanocomposite Membrane from Waste Materials for Treatment of Petroleum Industry Wastewater. J. Hazard. Mater. 2023, 442, 129955. [Google Scholar] [CrossRef]
- Hardian, R.; Cywar, R.M.; Chen, E.Y.X.; Szekely, G. Sustainable Nanofiltration Membranes Based on Biosourced Fully Recyclable Polyesters and Green Solvents. J. Membr. Sci. Lett. 2022, 2, 100016. [Google Scholar] [CrossRef]
- Park, S.-H.; Alammar, A.; Fulop, Z.; Pulido, B.A.; Nunes, S.P.; Szekely, G. Hydrophobic Thin Film Composite Nanofiltration Membranes Derived Solely from Sustainable Sources. Green Chem. 2021, 23, 1175–1184. [Google Scholar] [CrossRef]
- Meng, H.; Liang, H.; Xu, T.; Bai, J.; Li, C. Crosslinked Electrospinning Membranes with Contamination Resistant Properties for Highly Efficient Oil–Water Separation. J. Polym. Res. 2021, 28, 1–12. [Google Scholar] [CrossRef]










| Membranes | Filtration Type | Emulsion (Water-In-Oil or Oil/Water Mixtures | Pressure (Bar) | Water Contact Angle | Flux (Lm−2h−1) | Separation Efficiency | Ref. |
|---|---|---|---|---|---|---|---|
| Superhydrophobic isotactic polypropylene microporous membranes | Microfiltration | Oil Used: n-hexane, chloroform, and kerosene | 0.9 | 153° | 1230 ± 42 | - | [111] |
| PDMS/TA-Mn+/PI nanofibrous membrane | Microfiltration | Oil Used: dichloromethane, chloroform, 1,2-dichloroethane, bromobenzene, and tetrachloromethane | Gravity | 153.64 ± 1.6° | 6935 | 99% | [117] |
| PMMA/60PDMS nanofibrous membrane | Microfiltration | Oil Used: Hexane hexadecane Diesel, and Soybean | Gravity | 154° | 99.5% | [121] | |
| PML membrane | Microfiltration | Oil Used: Xylene, kerosene | Gravity | 153° ± 2° | 130–8800 | 99% | [115] |
| poly(vinylidene fluoride-co-hexafluoropropylene) (PVDF-HFP) nanofiber (PNF) membranes | Microfiltration | Oil Used: Dichloromethane, n-hexane, kerosene and toluene | Gravity | 155.0° | 9845 | 99.99% | [122] |
| Nano-structured ZnO/CuO mesh membrane | Microfiltration | Oil Used: Chloroform and n-hexane | Gravity | 161.2° ± 1.5° | above 2000 | >99.9% | [123] |
| BN-CuSA2 membrane | Microfiltration | Oil Used: dichloromethane kerosene | Gravity | 162.3° | 1667.63 | >95% | [124] |
| CNTs Reinforced Porous Electrospun Superhydrophobic Membrane | Microfiltration | Oil Used: dichloromethane, chloroform, 1,2-dichloroethane, | Gravity | 152° | 9270 | >99% | [125] |
| Carbon fiber membrane | Microfiltration | Oil Used: Dichloromethane, petroleum ether, ethyl acetate, carbon tetrachloride, toluene | Gravity | 155.9° | 3590 | 98% | [126] |
| polyurethane acrylate -based superhydrophobic membranes | Oil Used: Hexane | 0.02 | - | - | ∼97% | [127] |
| Materials/Membranes | Filtration Type | Emulsion (Oil-In-Water or Oil/Water Mixtures) | Pressure (Bar) | Water Contact Angle | Underwater Oil Contact Angle | Separation Efficiency | Ref. |
|---|---|---|---|---|---|---|---|
| polyacrylamide (PAM) hydrogel-coated mesh | Microfiltration | Oil used: Gasoline, diesel, vegetable oil, hexane, and petroleum ether | Gravity | 0° | 155.3° ± 1.8° | 99% | [137] |
| CuO@polyacrylic acid(PAA) | Microfiltration | Oil used: kerosene–water mixtures | Gravity | 0° | 160.4° | 99.90%, | [139] |
| Polysulfonamide/Polyvinylpyrrolidone Nanofibrous Membranes | Microfiltration | Oil used: n-hexane | Gravity | 0° | 150° | 99.7% | [141] |
| biomimetic BC/starch nanonet membrane. | Microfiltration | Oil used: diesel, vegetable oil, hexane, petroleum ether, and silicon oil | Gravity | 0° | 150° | 99.996% | [140] |
| TiO2@GO/PEN FCM | Microfiltration | Oil used: petroleum ether | 0.4 | 0° | 162.5° | 99% | [138] |
| FOGE-TA-SSM | Microfiltration | Oil used: kerosene, cyclohexane, n-hexane, n-dodecane, and petroleum ether. | Gravity | 0 | 155 | 99% | [144] |
| AL/RGO@PDA | Microfiltration | Oil used: soybean oil, engine oil, n-hexadecane, kerosene, and trichloromethane | - | 0° | 151° | 99.10% | [142] |
| PVP-UiO-66-NH2/PAN | Microfiltration | Oil used: n-hexane | Gravity | 0° | 165.4° | 99.2% | [145] |
| The polyaniline-coated alumina membranes | Microfiltration | Oil used: diesel | 1.5 | 0° | 150° | 97% | [58] |
| CFHP/PDA-coated membrane | Microfiltration | Oil used: dichloromethane (DCM), petroleum ether, chloroform, gasoline, hexane, and methylbenzene | Gravity | 0° | 150° | 99.96% | [146] |
| ceramic membrane with TiO2 nanowire | Microfiltration | Oil used: Diesel | 0.1–0.3 | <5° | 158° | 97% | [147] |
| MXene@TiO2/PEN membrane | Microfiltration | Oil used: Isooctane | 0.4 | 0° | 155° | 99.13% | [148] |
| CS-TPP@PDA@nylon membrane | Microfiltration | Oil used: Methyl silicone oil, colza oil, or diesel oil | Gravity | 0° | 179.6° | 99.94% | [143] |
| PVDF@ZnO membrane | Microfiltration | Oil used: n-hexane, petrol, toluene, and diesel | Gravity | 0° | 162° | 99% | [149] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baig, N.; Sajid, M.; Salhi, B.; Abdulazeez, I. Special Wettable Membranes for Oil/Water Separations: A Brief Overview of Properties, Types, and Recent Progress. Colloids Interfaces 2023, 7, 11. https://doi.org/10.3390/colloids7010011
Baig N, Sajid M, Salhi B, Abdulazeez I. Special Wettable Membranes for Oil/Water Separations: A Brief Overview of Properties, Types, and Recent Progress. Colloids and Interfaces. 2023; 7(1):11. https://doi.org/10.3390/colloids7010011
Chicago/Turabian StyleBaig, Nadeem, Muhammad Sajid, Billel Salhi, and Ismail Abdulazeez. 2023. "Special Wettable Membranes for Oil/Water Separations: A Brief Overview of Properties, Types, and Recent Progress" Colloids and Interfaces 7, no. 1: 11. https://doi.org/10.3390/colloids7010011