N Absorption, Transport, and Recycling in Nodulated Soybean Plants by Split-Root Experiment Using 15N-Labeled Nitrate
Abstract
:1. Introduction
2. Materials and Methods
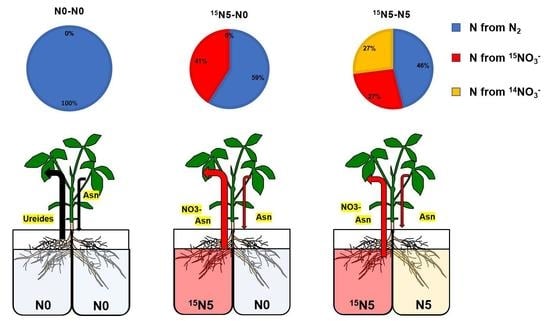
2.1. Split-Root Experiment Supplied with 15N-Labeled Nitrate from One Half-Root
2.2. The Effects of Split-Root Treatments on the Principal N Metabolites in Each Part of Soybean Plants
3. Results
3.1. 15N Translocation from the Half-Roots Supplied with 15NO3− to the Other Parts
3.1.1. N Content in Each Part of Soybean Plants
3.1.2. N Content from 15N-Labeled Nitrate in Each Part of Soybean Plants
3.1.3. Percentage of N Derived from 15N-Labeled Nitrate in Each Part of Soybean Plants
3.1.4. Percentage Distribution of N Derived from 15N-Labeled Nitrate in Each Part of Soybean Plants
3.2. Comparisons of Principal N Metabolites in Each Part of the Plants
3.2.1. Nitrate Concentration in Each Part of Soybean Plants
3.2.2. N Concentrations of Major N Metabolites in Leaves, Stems, and Basal Roots
3.2.3. N Concentrations of Principal N Metabolites in the Upper and Lower Roots
3.2.4. N Concentrations of Principal N Compounds in the Upper and Lower Nodules
4. Discussion
4.1. Changes in Total N and N Derived from 15N-Labeled Nitrate with Split-Root Systems
4.2. Transport Pathways of Nitrate Absorbed in the Half-Roots Supplied with 15NO3− to the Shoot and the Opposite Side of Roots
4.3. Changes in Amides, Amino Acids, and Ureides in Each Part of the Plants with Split-Root Systems
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Baslam, M.; Mitsui, T.; Sueyoshi, K.; Ohyama, T. Recent advances in carbon and nitrogen metabolism in C3 plants. Int. J. Mol. Sci. 2021, 22, 318. [Google Scholar] [CrossRef]
- Forde, B.G. Local and long-range signaling pathways regulating plant responses to nitrate. Annu. Rev. Plant Biol. 2002, 53, 203–224. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.J.; Fan, X.R.; Orsel, M.; Smith, S.J.; Wells, D.M. Nitrate transport and signaling. J. Exp. Bot. 2007, 58, 2297–2306. [Google Scholar] [CrossRef] [PubMed]
- Crawford, N.M.; Kahn, M.L.; Leustek, T.; Long, S.R. Nitrogen and sulfur. In Biochemistry and Molecular Biology of Plants; Buchanan, B., Gruissem, W., Jones, R., Eds.; American Society of Plant Physiologist: Rockville, MD, USA, 2000; pp. 786–849. [Google Scholar]
- Oghoghorie, C.G.O.; Pate, J.S. Exploration of the nitrogen transport system of a nodulated legume using 15N. Planta 1972, 104, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Fujita, K.; Terasawa, H. Growth and dinitrogen fixation of soybean root system affected by partial exposure to nitrate. Soil Sci. Plant Nutr. 1985, 31, 637–645. [Google Scholar] [CrossRef] [Green Version]
- Cho, M.-J.; Harper, J.E. Effect of localized nitrate application on isoflavonoid concentration and nodulation in split-root systems of wild-type and nodulation-mutant soybean plants. Plant Physiol. 1991, 95, 1106–1112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carroll, B.J.; Gresshoff, P.M. Nitrate Inhibition of Nodulation and Nitrogen Fixation in White Clover. Z. Pflanzenphysiol. 1983, 110, 77–88. [Google Scholar] [CrossRef]
- Arnone, J.A.; Kohls, S.J.; Baker, D.D. Nitrate effects on nodulation and nitrogenase activity of actinorhizal Casuarina studied in split-root systems. Soil Biol. Biochem. 1994, 26, 599–606. [Google Scholar] [CrossRef]
- Jeudy, C.; Ruffel, S.; Freixes, S.; Tillard, P.; Santoni, A.L.; Morel, S.; Journet, E.-P.; Duc, G.; Gojon, A.; Lepetit, M.; et al. Adaptation of Medicago truncatula to nitrogen limitation is modulated via local and systemic nodule developmental responses. New Phytol. 2009, 185, 817–828. [Google Scholar] [CrossRef]
- Daimon, H.; Yoshioka, M. Responses of Root Nodule Formation and Nitrogen Fixation Activity to Nitrate in a Split-Root System in Peanut (Arachis hypogaea L.). J. Agron. Crop Sci. 2008, 187, 89–95. [Google Scholar] [CrossRef]
- Fujikake, H.; Yamazaki, A.; Ohtake, N.; Sueyoshi, K.; Matsuhashi, S.; Ito, T.; Mizuniwa, C.; Kume, T.; Hashimoto, S.; Ishioka, N.S.; et al. Quick and reversible inhibition of soybean root nodule growth by nitrate involves a decrease in sucrose supply to nodules. J. Exp. Bot. 2003, 54, 1379–1388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, A.H.; Harper, J.E. Nitrate effect on nodulation of soybean by Bradyrhizobium japonicum. Crop Sci. 1985, 25, 497–501. [Google Scholar] [CrossRef]
- Harper, J.E. Nitrogen metabolism. In Soybeans: Improvement, Production, and Uses, 2nd ed.; Wilcox, J.R., Ed.; Agronomy Monograph no.16; American Society of Agronomy: Madison, WI, USA, 1987; pp. 497–533. [Google Scholar]
- Streeter, J. Inhibition of legume nodule formation and N2 fixation by nitrate. CRC Crit. Rev. Plant Sci. 1988, 7, 1–23. [Google Scholar] [CrossRef]
- Imsande, J. Inhibition of nodule development in soybean by nitrate or reduced nitrogen. J. Exp. Bot. 1986, 37, 348–355. [Google Scholar] [CrossRef]
- Ohyama, T.; Fujikake, H.; Yashima, H.; Tanabata, S.; Ishikawa, S.; Sato, T.; Nishiwaki, T.; Ohtake, N.; Sueyoshi, K.; Ishii, S.; et al. Effect of Nitrate on Nodulation and Nitrogen Fixation of soybean. In Soybean Physiology and Biology; El-Shemy, H.A., Ed.; InTech: Rijeka, Croatia, 2012; pp. 333–364. [Google Scholar] [CrossRef] [Green Version]
- Yashima, H.; Fujikake, H.; Sato, T.; Ohtake, N.; Sueyoshi, K.; Ohyama, T. Systemic and local effects of long-term application of nitrate on nodule growth and N2 fixation in soybean (Glycine max [L.] Merr.). Soil Sci. Plant Nutr. 2003, 49, 825–834. [Google Scholar] [CrossRef]
- Yashima, H.; Fujikake, H.; Yamazaki, A.; Ito, S.; Sato, T.; Tewari, K.; Ohtake, N.; Sueyoshi, K.; Takahashi, Y.; Ohyama, T. Long-term effect of nitrate application from lower part of roots on nodulation and N2 fixation in upper part of roots of soybean (Glycine max (L.) Merr.) in two-layered pot experiment. Soil Sci. Plant Nutr. 2005, 51, 981–990. [Google Scholar] [CrossRef] [Green Version]
- Harper, J.E.; Gibson, A.H. Differential nodulation tolerance to nitrate among legume species. Crop Sci. 1984, 24, 797–801. [Google Scholar] [CrossRef]
- Davidson, I.A.; Robson, M.J. Effect of contrasting patterns of nitrate application on the nitrate uptake, N2-fixation, nodulation and growth of white clover. Ann. Bot. 1986, 57, 331–338. [Google Scholar] [CrossRef]
- Fujikake, H.; Yashima, H.; Sato, T.; Norikuni, O.; Sueyoshi, K.; Ohyama, T. Rapid and reversible nitrate inhibition of nodule growth and N2 fixation activity in soybean (Glycine max (L.) Merr.). Soil Sci. Plant Nutr. 2002, 48, 211–217. [Google Scholar] [CrossRef]
- Saito, A.; Tanabata, S.; Tanabata, T.; Tajima, S.; Ueno, M.; Ishikawa, S.; Ohtake, N.; Sueyoshi, K.; Ohyama, T. Effect of nitrate on nodule and root growth of soybean (Glycine max (L.) Merr.). Int. J. Mol. Sci. 2014, 15, 4464–4480. [Google Scholar] [CrossRef] [Green Version]
- Tanabata, S.; Tanabata, T.; Saito, A.; Tajima, S.; Watanabe, S.; Ishikawa, K.; Ohtake, N.; Sueyoshi, K.; Ohyama, T. Computational image analysis method for measuring size of nodule growth in soybean. Jpn. J. Soil Sci. Plant Nutr. 2014, 85, 43–47. [Google Scholar]
- Ishikawa, S.; Ono, Y.; Ohtake, N.; Sueyoshi, K.; Tanabata, S.; Ohyama, T. Transcriptome and metabolome analysis reveal that nitrate strongly promotes nitrogen and carbon metabolism in soybean roots, but tend to repress it in nodules. Plants 2018, 7, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamashita, N.; Tanabata, S.; Ohtake, N.; Sueyoshi, K.; Sato, T.; Higuchi, K.; Saito, A.; Ohyama, T. Effects of Different Chemical Forms of Nitrogen on the Quick and Reversible Inhibition of Soybean Nodule Growth and Nitrogen Fixation Activity. Front. Plant Sci. 2019, 10, 131. [Google Scholar] [CrossRef]
- Laine, P.; Ourry, A.; Boucaud, J. Shoot control of nitrate uptake rates by roots of Brassica napus L.: Effects of localized nitrate supply. Planta 1995, 196, 77–83. [Google Scholar] [CrossRef]
- Cerezo, M.; Tillard, P.; Filleur, S.; Munos, S.; Daniel-Vedele, F.; Gojon, A. Alterations of the regulation of root NO3− uptake are associated with the mutationof Nrt2.1 and Nrt2.2 genes in Arabidopsis. Plant Physiol. 2001, 127, 262–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filleur, S.; Dorbe, M.F.; Cerezo, M.; Orsel, M.; Granier, F.; Gojon, A.; Daniel-Vedele, F. An Arabidopsis T-DNA mutant affected in Nrt2 genes is impaired in nitrate uptake. FEBS Lett. 2001, 489, 220–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohyama, T.; Ueno, M.; Ono, Y.; Ohtake, N.; Sueyoshi, K.; Sato, T.; Tanabata, S. Recycling of nitrogen from shoots to underground parts in hypernodulation mutant lines of soybean by split-root experiment. Bull. Facul. Agric. Niigata Univ. 2017, 70, 1–8. [Google Scholar]
- Mizukoshi, K.; Nishiwaki, T.; Ohtake, N.; Minagawa, R.; Ikarashi, T.; Ohyama, T. Nitrate transport pathway into soybean nodules traced by tungstate and 15NO3−. Soil Sci. Plant Nutr. 1995, 41, 75–88. [Google Scholar] [CrossRef]
- Li, S.; Xiao, F.; Yang, D.; Lyu, X.; Ma, C.; Dong, S.; Yan, C.; Gong, Z. Nitrate transport and distribution in soybean plants with dual-root systems. Front. Plant Sci. 2021, 12, 661054. [Google Scholar] [CrossRef]
- Ohyama, T.; Kawai, S. Nitrogen assimilation and transport in soybean leaves: Investigation by petiole girdling treatment. Soil Sci. Plant Nutr. 1983, 29, 227–231. [Google Scholar] [CrossRef] [Green Version]
- Cooper, H.D.; Clarkson, D.T. Cycling of amino nitrogen and other nutrients between shoots and roots in cereals: A possible mechanism integrating shoot and root in the regulation of nutrient uptake. J. Exp. Bot. 1989, 40, 753–762. [Google Scholar] [CrossRef]
- Imsande, J.; Touraine, B. N demand and the regulation of nitrate uptake. Plant Physiol. 1994, 105, 3–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuller, K.A.; Minchin, F.R.; Gresshoff, P.M. Nitrogenase activity and oxygen diffusion in nodules of soybean cv. Bragg and a supernodulating mutant: Effects of nitrate. J. Exp. Bot. 1988, 39, 865–877. [Google Scholar] [CrossRef]
- Vidal, E.A.; Araus, V.; Lu, C.; Parry, G.; Green, P.J.; Coruzzi, G.M.; Gutiérrez, R.A. Nitrate-responsivemiR393/AFB3 regulatory module controls root system architecture in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2010, 107, 4477–4482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peuke, A.D.; Hartung, W.; Jeschke, W.D. The uptake and flow of C, N and ions between roots and shoots in Ricinus communis L. II. Grown with low or high nitrate supply. J. Exp. Bot. 1994, 45, 733–740. [Google Scholar] [CrossRef]
- Tillard, P.; Passama, L.; Gojon, A. Are phloem amino acids involved in the shoot to root control of NO3−-uptake in Ricinus communis plants? J. Exp. Bot. 1998, 49, 1371–1379. [Google Scholar] [CrossRef]
- Serraj, R.; Vadez, V.; Denison, R.F.; Sinclair, T.R. Involvement of ureides in nitrogen fixation inhibition in soybean. Plant Physiol. 1999, 119, 289–296. [Google Scholar] [CrossRef] [Green Version]
- Vadez, V.; Sinclair, T.R.; Serraj, R. Asparagine and ureide accumulation in nodules and shoots as feedback inhibitors of N2 fixation in soybean. Physiol. Plant. 2000, 110, 215–223. [Google Scholar] [CrossRef]
- Vessey, J.K.; Walsh, K.B.; Layzell, D.B. Can a limitation in phloem supply to nodules account for the inhibitory effect of nitrate on nitrogenase activity? Physiol. Plant. 1988, 74, 137–146. [Google Scholar] [CrossRef]
- Gordon, A.J.; Skøt, L.; James, C.L.; Minchin, F.R. Short-term metabolic responses of soybean root nodules to nitrate. J. Exp. Bot. 2002, 53, 423–428. [Google Scholar] [CrossRef]
- Gogorcena, Y.; Gordon, A.J.; Escuredo, P.R.; Minchin, F.R.; Witty, J.F.; Moran, J.F.; Becana, M. N2 fixation, carbon metabolism, and oxidative damage in nodules of dark-stressed common bean plants. Plant Physiol. 1997, 113, 1193–1201. [Google Scholar] [CrossRef] [PubMed]








| a. 15N Distribution in Leaves, Stems, and Basal Roots. | |||||
| Treatment | Days | Leaves | Stems | Basal Roots | |
| 15N5-N0 | 2 DAT | 45.6 (1.1) | 10.2 (0.3) | 2.3 (0.2) | |
| 15N5-N5 | 2 DAT | 47.0 (0.9) | 10.4 (0.6) | 3.7 (0.6) | |
| 15N5-N0 | 4 DAT | 57.8 (0.6) | 10.3 (0.4) | 3.8 (0.1) | |
| 15N5-N5 | 4 DAT | 55.2 (2.3) | 10.8 (1.6) | 4.5 (0.3) | |
| b. 15N Distribution in Nodules and Roots in 15N-Labeled Pot [L]. | |||||
| Treatment | Days | U-Nodules | L-Nodules | U-Roots | L-Roots |
| 15N5-N0 | 2 DAT | 1.04 (0.32) | 3.00 (0.74) | 3.25 (0.38) | 32.0 (0.6) |
| 15N5-N5 | 2 DAT | 1.07 (0.22) | 1.42 (0.50) | 4.10 (1.54) | 29.6 (1.6) |
| 15N5-N0 | 4 DAT | 0.73 (0.13) | 0.71 (0.29) | 2.48 (0.41) | 22.1 (1.0) |
| 15N5-N5 | 4 DAT | 0.75 (0.11) | 0.69 (0.15) | 2.92 (0.28) | 22.7 (1.4) |
| c. 15N Distribution in Nodules and Roots in Non-Labeled Pot [R]. | |||||
| Treatment | Days | U-Nodules | L-Nodules | U-Roots | L-Roots |
| 15N5-N0 | 2 DAT | 0.21 (0.06) | 0.34 (0.06) | 0.35 (0.06) | 1.68 (0.21) |
| 15N5-N5 | 2 DAT | 0.35 (0.11) | 0.15 (0.07) | 0.37 (0.05) | 1.87 (0.15) |
| 15N5-N0 | 4 DAT | 0.25 (0.01) | 0.29 (0.06) | 0.29 (0.03) | 1.26 (0.06) |
| 15N5-N5 | 4 DAT | 0.13 (0.02) | 0.04 (0.02) | 0.32 (0.05) | 1.88 (0.39) |
| Data are means (standard error). N = 4 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doi, M.; Higuchi, K.; Saito, A.; Sato, T.; Ohyama, T. N Absorption, Transport, and Recycling in Nodulated Soybean Plants by Split-Root Experiment Using 15N-Labeled Nitrate. Nitrogen 2022, 3, 636-651. https://doi.org/10.3390/nitrogen3040042
Doi M, Higuchi K, Saito A, Sato T, Ohyama T. N Absorption, Transport, and Recycling in Nodulated Soybean Plants by Split-Root Experiment Using 15N-Labeled Nitrate. Nitrogen. 2022; 3(4):636-651. https://doi.org/10.3390/nitrogen3040042
Chicago/Turabian StyleDoi, Maria, Kyoko Higuchi, Akihiro Saito, Takashi Sato, and Takuji Ohyama. 2022. "N Absorption, Transport, and Recycling in Nodulated Soybean Plants by Split-Root Experiment Using 15N-Labeled Nitrate" Nitrogen 3, no. 4: 636-651. https://doi.org/10.3390/nitrogen3040042