Toxic External Exposure Leading to Ocular Surface Injury
Abstract
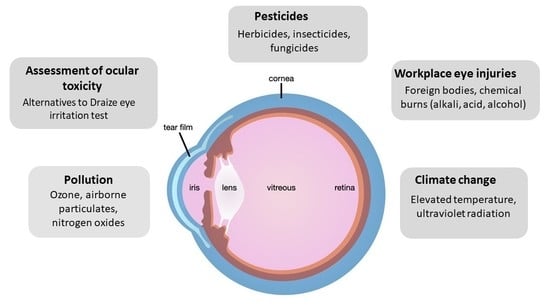
:1. Introduction
2. Assessments of Ocular Toxicity
2.1. The Draize Eye Test
2.2. In Vitro Testing: Reconstructed Human Cornea-like Epithelium (RhCE)
2.3. In Silico Models
3. Pollution Effects
3.1. Ozone
3.2. Airborne Particulate Matter
3.3. Nitrogen Oxides
3.4. Combined Pollutants
4. Air Bag Deployment
5. Pesticide Exposure
5.1. Pesticide Overview
5.2. Herbicides and Insecticides
5.3. Fungicides
6. Workplace Ocular Injuries
6.1. Overview
6.2. Foreign Object Injuries
6.3. Chemical Injuries
6.4. Preventing Damage from Chemicals and Foreign Bodies
7. Climate Change
7.1. Key Features of Climate Change
7.2. Elevated Global Temperature and Increased Frequency of Extreme Heat Events
7.3. Air Quality
7.4. Increased Ultraviolet (UV) Radiation
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fischer, I.; Milton, C.; Wallace, H. Toxicity testing is evolving! Toxicol. Res. 2020, 9, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Chuprina, A.; Lukin, O.; Demoiseaux, R.; Buzko, A.; Shivanyuk, A. Drug- and lead-likeness, target class, and molecular diversity analysis of 7.9 million commercially available organic compounds provided by 29 suppliers. J. Chem. Inf. Model. 2010, 50, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Haring, R.S.; Sheffield, I.D.; Channa, R.; Canner, J.K.; Schneider, E.B. Epidemiologic Trends of Chemical Ocular Burns in the United States. JAMA Ophthalmol. 2016, 134, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Prior, H.; Casey, W.; Kimber, I.; Whelan, M.; Sewell, F. Reflections on the Progress towards Non-Animal Methods for Acute Toxicity Testing of Chemicals. Regul. Toxicol. Pharmacol. 2019, 102, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Fitzhugh, O.G.; Woodard, G. The toxicities of compounds related to 2,3-dimercaptopropanol (BAL) with a note on their relative therapeutic efficiency. J. Pharmacol. Exp. Ther. 1946, 87, 23–27. [Google Scholar]
- Draize, J.H.; Woodard, G.; Calvery, H.O. Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J. Pharmacol. Exp. Ther. 1944, 82, 377–390. [Google Scholar]
- Wilhelmus, K.R. The Draize eye test. Surv. Ophthalmol. 2001, 45, 493–515. [Google Scholar] [CrossRef]
- Barile, F.A. Validating and troubleshooting ocular in vitro toxicology tests. J. Pharm. Toxicol. Methods 2010, 61, 136–145. [Google Scholar] [CrossRef] [Green Version]
- Vinardell, M.P.; Mitjans, M. Alternative methods for eye and skin irritation tests: An overview. J. Pharm. Sci. 2008, 97, 46–59. [Google Scholar] [CrossRef]
- Lieto, K.; Skopek, R.; Lewicka, A.; Stelmasiak, M.; Klimaszewska, E.; Zelent, A.; Szymański, Ł.; Lewicki, S. Looking into the Eyes-In Vitro Models for Ocular Research. Int. J. Mol. Sci. 2022, 23, 9158. [Google Scholar] [CrossRef]
- Curren, R.D.; Harbell, J.W. Ocular safety: A silent (in vitro) success story. Altern. Lab. Anim. 2002, 30, 69–74. [Google Scholar] [CrossRef]
- Bonneau, N.; Baudouin, C.; Réaux-Le Goazigo, A.; Brignole-Baudouin, F. An overview of current alternative models in the context of ocular surface toxicity. J. Appl. Toxicol. 2022, 42, 718–737. [Google Scholar] [CrossRef]
- Chacón, M.; Vázquez, N.; Persinal-Medina, M.; Alonso-Alonso, S.; Alcalde, I.; Merayo-Lloves, J.; Meana, Á. In-house performance assessment of 3D QobuR-Reconstructed Human Cornea-Like Epithelium (RhCE) for the evaluation of eye hazard. Toxicol. In Vitro 2022, 82, 105390. [Google Scholar] [CrossRef]
- Narda, M.; Ramos-Lopez, D.; Mun, G.; Valderas-Martinez, P.; Granger, C. Three-tier testing approach for optimal ocular tolerance sunscreen. Cutan. Ocul. Toxicol. 2019, 38, 212–220. [Google Scholar] [CrossRef]
- Matsuda, S.; Hisama, M.; Shibayama, H.; Itou, N.; Iwaki, M. Application of the reconstructed rabbit corneal epithelium model to assess the in-vitro eye irritant test of chemicals. Yakugaku Zasshi 2009, 129, 1113–1120. [Google Scholar] [CrossRef] [Green Version]
- Kaluzhny, Y.; Kandárová, H.; Hayden, P.; Kubilus, J.; d’Argembeau-Thornton, L.; Klausner, M. Development of the EpiOcular(TM) eye irritation test for hazard identification and labelling of eye irritating chemicals in response to the requirements of the EU cosmetics directive and REACH legislation. Altern. Lab. Anim. 2011, 39, 339–364. [Google Scholar] [CrossRef]
- Alépée, N.; Leblanc, V.; Adriaens, E.; Grandidier, M.H.; Lelièvre, D.; Meloni, M.; Nardelli, L.; Roper, C.S.; Santirocco, E.; Toner, F.; et al. Multi-laboratory validation of SkinEthic HCE test method for testing serious eye damage/eye irritation using liquid chemicals. Toxicol. In Vitro 2016, 31, 43–53. [Google Scholar] [CrossRef]
- Stern, M.; Klausner, M.; Alvarado, R.; Renskers, K.; Dickens, M. Evaluation of the EpiOcular((TM)) tissue model as an alternative to the Draize eye irritation test. Toxicol. In Vitro 1998, 12, 455–461. [Google Scholar] [CrossRef]
- Kandarova, H.; Letasiova, S.; Adriaens, E.; Guest, R.; Willoughby, J.A., Sr.; Drzewiecka, A.; Gruszka, K.; Alépée, N.; Verstraelen, S.; Van Rompay, A.R. CON4EI: EpiOcular™ Eye Irritation Test (EpiOcular™ EIT) for hazard identification and labelling of eye irritating chemicals. Toxicol. In Vitro 2018, 49, 21–33. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Decker, T.; Lohmann-Matthes, M.L. A quick and simple method for the quantitation of lactate dehydrogenase release in measurements of cellular cytotoxicity and tumor necrosis factor (TNF) activity. J. Immunol. Methods 1988, 115, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Cotovio, J.; Grandidier, M.H.; Portes, P.; Roguet, R.; Rubinstenn, G. The in vitro skin irritation of chemicals: Optimisation of the EPISKIN prediction model within the framework of the ECVAM validation process. Altern. Lab. Anim. 2005, 33, 329–349. [Google Scholar] [CrossRef] [PubMed]
- Ichijima, H.; Ohashi, J.; Cavanagh, H.D. Effect of contact-lens-induced hypoxia on lactate dehydrogenase activity and isozyme in rabbit cornea. Cornea 1992, 11, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Maurer, J.K.; Parker, R.D.; Jester, J.V. Extent of initial corneal injury as the mechanistic basis for ocular irritation: Key findings and recommendations for the development of alternative assays. Regul. Toxicol. Pharm. 2002, 36, 106–117. [Google Scholar] [CrossRef]
- Eskes, C.; Bessou, S.; Bruner, L.; Curren, R.; Harbell, J.; Jones, P.; Kreiling, R.; Liebsch, M.; McNamee, P.; Pape, W.; et al. Eye Irritation. Altern. Lab. Anim. 2005, 33, 47–81. [Google Scholar] [CrossRef]
- Lebrun, S.; Nguyen, L.; Chavez, S.; Chan, R.; Le, D.; Nguyen, M.; Jester, J.V. Same-chemical comparison of nonanimal eye irritation test methods: Bovine corneal opacity and permeability, EpiOcular™, isolated chicken eye, ocular Irritection®, OptiSafe™, and short time exposure. Toxicol. In Vitro 2021, 72, 105070. [Google Scholar] [CrossRef]
- Doucet, O.; Lanvin, M.; Thillou, C.; Linossier, C.; Pupat, C.; Merlin, B.; Zastrow, L. Reconstituted human corneal epithelium: A new alternative to the Draize eye test for the assessment of the eye irritation potential of chemicals and cosmetic products. Toxicol. In Vitro 2006, 20, 499–512. [Google Scholar] [CrossRef]
- Abbate, I.; Zappulla, C.; Santonocito, M.; Viola, S.; La Rosa, L.R.; De Pasquale, G.; Caviola, E.; Meloni, M.; Curatolo, M.C.; Mazzone, M.G. Preclinical study of a new matrix to help the ocular surface in dry eye disease. Exp. Eye Res. 2022, 222, 109168. [Google Scholar] [CrossRef]
- Leblanc, V.; Yokota, M.; Grandidier, M.H.; Yoshida, D.; Adriaens, E.; Cotovio, J.; Kyoutani, D.; Alépée, N. SkinEthic™ HCE Eye Irritation Test: Similar performance demonstrated after long distance shipment and extended storage conditions. Toxicol. In Vitro 2019, 54, 202–214. [Google Scholar] [CrossRef]
- Alépée, N.; Grandidier, M.H.; Teluob, S.; Amaral, F.; Caviola, E.; De Servi, B.; Martin, S.; Meloni, M.; Nardelli, L.; Pasdelou, C.; et al. Validation of the SkinEthic HCE Time-to-Toxicity test method for eye hazard classification of chemicals according to UN GHS. Toxicol. In Vitro 2022, 80, 105319. [Google Scholar] [CrossRef]
- United Nations. Globally Harmonized System of Classification and Labelling of Chemicals (GHS); United Nations: New York, NY, USA; Geneva, Switzerland, 2019; Available online: https://www.unece.org/fileadmin/DAM/trans/danger/publi/ghs/ghs_rev08/ST-SG-AC10-30-Rev8e.pdf (accessed on 5 January 2023).
- Alépée, N.; Leblanc, V.; Grandidier, M.H.; Teluob, S.; Viricel, A.; Adriaens, E.; Michaut, V. SkinEthic HCE Time-to-Toxicity on solids: A test method for distinguishing chemicals inducing serious eye damage, eye irritation and not requiring classification and labelling. Toxicol. In Vitro 2021, 75, 105203. [Google Scholar] [CrossRef]
- Deeb, O.; Goodarzi, M. In silico quantitative structure toxicity relationship of chemical compounds: Some case studies. Curr. Drug Saf. 2012, 7, 289–297. [Google Scholar] [CrossRef]
- Valerio, L.G., Jr. In silico toxicology for the pharmaceutical sciences. Toxicol. Appl. Pharmacol. 2009, 241, 356–370. [Google Scholar] [CrossRef]
- Valerio, L.G., Jr. In silico toxicology models and databases as FDA Critical Path Initiative toolkits. Hum. Genom. 2011, 5, 200–207. [Google Scholar] [CrossRef] [Green Version]
- Sinha, M.; Dhawan, A.; Parthasarathi, R. In silico approaches in predictive genetic toxicology. Methods Mol. Biol. 2019, 2031, 351–373. [Google Scholar] [CrossRef]
- Rim, K.T. In silico prediction of toxicity and its applications for chemicals at work. Toxicol. Environ. Health Sci. 2020, 12, 191–202. [Google Scholar] [CrossRef]
- Chinen, K.; Malloy, T. QSAR Use in REACH analyses of alternatives to predict human health and environmental toxicity of alternative chemical substances. Integr. Environ. Assess. Manag. 2020, 16, 745–760. [Google Scholar] [CrossRef]
- Fourches, D.; Muratov, E.; Tropsha, A. Trust, but verify: On the importance of chemical structure curation in cheminformatics and QSAR modeling research. J. Chem. Inf. Model. 2010, 50, 1189–1203. [Google Scholar] [CrossRef]
- Pope, C.A.; Dockery, D.W. Health effects of fine particulate air pollution: Lines that connect. J. Air Waste Manag. Assoc. 2006, 5, 709–742. [Google Scholar] [CrossRef]
- Burnett, R.; Chen, H.; Szyszkowicz, M.; Fann, N.; Hubbell, B.; Pope, C.A., 3rd; Apte, J.S.; Brauer, M.; Cohen, A.; Weichenthal, S.; et al. Global estimates of mortality associated with long-term exposure to outdoor fine particulate matter. Proc. Natl. Acad. Sci. USA 2018, 115, 9592–9597. [Google Scholar] [CrossRef] [Green Version]
- Anenberg, S.C.; West, J.J.; Fiore, A.M.; Jaffe, D.A.; Prather, M.J.; Bergmann, D.; Cuvelier, K.; Dentener, F.J.; Duncan, B.N.; Gauss, M. Intercontinental impacts of ozone pollution on human mortality. Environ. Sci. Technol. 2009, 43, 6482–6487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.C.; Chiu, C.C.; Lee, P.Y.; Chen, K.J.; He, C.X.; Hsu, S.K.; Cheng, K.C. The adverse effects of air pollution on the eye: A review. Int. J. Environ. Res. Public Health 2022, 19, 1186. [Google Scholar] [CrossRef] [PubMed]
- Boogaard, H.; Patton, A.P.; Atkinson, R.W.; Brook, J.R.; Chang, H.H.; Crouse, D.L.; Fussell, J.C.; Hoek, G.; Hoffmann, B.; Kappeler, R.; et al. Long-term exposure to traffic-related air pollution and selected health outcomes: A systematic review and meta-analysis. Environ. Int. 2022, 164, 107262. [Google Scholar] [CrossRef] [PubMed]
- Orru, H.; Ebi, K.L.; Forsberg, B. The interplay of climate change and air pollution on health. Curr. Environ. Health Rep. 2017, 4, 504–513. [Google Scholar] [CrossRef]
- Mao, M.; Rao, L.; Jiang, H.; He, S.; Zhang, X. Air pollutants in metropolises of eastern coastal China. Int. J. Environ. Res. Public Health 2022, 19, 15332. [Google Scholar] [CrossRef]
- Koh, S.; Tung, C.I.; Inoue, Y.; Jhanji, V. Effects of tear film dynamics on quality of vision. Br. J. Ophthalmol. 2018, 102, 1615–1620. [Google Scholar] [CrossRef]
- Jung, S.J.; Jodhbir, S.M.; Tong, L. Effects of environment pollution on the ocular surface. Ocul Surf. 2018, 16, 198–205. [Google Scholar] [CrossRef]
- Zhang, J.J.; Wei, Y.; Fang, Z. Ozone pollution: A major health hazard worldwide. Front. Immunol. 2019, 10, 2518. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Kim, E.K.; Kim, H.Y.; Kim, T.I. Effects of exposure to ozone on the ocular surface in an experimental model of allergic conjunctivitis. PLoS ONE 2017, 12, e0169209. [Google Scholar] [CrossRef] [Green Version]
- Seen, S.; Tong, L. Dry eye disease and oxidative stress. Acta Ophthalmol. 2018, 96, e412–e420. [Google Scholar] [CrossRef] [Green Version]
- Dogru, M.; Kojima, T.; Simsek, C.; Tsubota, K. Potential role of oxidative stress in ocular surface inflammation and dry eye disease. Investig. Ophthalmol. Vis. Sci. 2018, 59, DES163–DES168. [Google Scholar] [CrossRef] [Green Version]
- Pan, S.C.; Huang, C.C.; Chin, W.S.; Chen, B.Y.; Chan, C.C.; Guo, Y.L. Association between air pollution exposure and diabetic retinopathy among diabetics. Environ. Res. 2020, 18. [Google Scholar] [CrossRef]
- Nunez, Y.; Boehme, A.K.; Goldsmith, J.; Li, M.; van Donkelaar, A.; Weisskopf, M.G.; Re, D.B.; Martin, R.V.; Kioumourtzoglou, M.A. PM2.5 composition and disease aggravation in amyotrophic lateral sclerosis: An analysis of long-term exposure to components of fine particulate matter in New York State. Environ. Epidemiol. 2022, 6, e204. [Google Scholar] [CrossRef]
- Pan, S.; Qiu, Y.; Li, M.; Yang, Z.; Liang, D. Recent developments in the determination of PM2.5 chemical composition. Bull. Environ. Contam. Toxicol. 2022, 108, 819–823. [Google Scholar] [CrossRef]
- Morishita, M.; Bard, R.L.; Wang, L.; Das, R.; Dvonch, J.T.; Spino, C.; Mukherjee, B.; Sun, Q.; Harkema, J.R.; Rajagopalan, S.; et al. The characteristics of coarse particulate matter air pollution associated with alterations in blood pressure and heart rate during controlled exposures. J. Expo. Sci. Environ. Epidemiol. 2015, 25, 153–159. [Google Scholar] [CrossRef] [Green Version]
- Prasannavenkatesh, R.; Andimuthu, R.; Kandasamy, P.; Rajadurai, G.; Kumar, D.S.; Radhapriya, P.; Ponnusamy, M. Assessment of population exposure to coarse and fine particulate matter in the urban areas of Chennai, India. Sci. World J. 2015, 2015, 643714. [Google Scholar] [CrossRef] [Green Version]
- Anenberg, S.C.; West, J.J.; Yu, H.; Chin, M.; Schulz, M.; Bergmann, D.; Bey, I.; Bian, H.; Diehl, T.; Fiore, A. Impacts of intercontinental transport of anthropogenic fine particulate matter on human mortality. Air Qual. Atmos. Health 2014, 7, 369–379. [Google Scholar] [CrossRef]
- Adar, S.D.; Filigrana, P.A.; Clements, N.; Peel, J.L. Ambient coarse particulate matter and human health: A systematic review and meta-analysis. Curr. Environ. Health Rep. 2014, 1, 258–274. [Google Scholar] [CrossRef] [Green Version]
- Tan, G.; Li, J.; Yang, Q.; Wu, A.; Qu, D.Y.; Wang, Y.; Ye, L.; Bao, J.; Shao, Y. Air pollutant particulate matter 2.5 induces dry eye syndrome in mice. Sci. Rep. 2018, 8, 17828. [Google Scholar] [CrossRef] [Green Version]
- Song, S.J.; Hyun, S.W.; Lee, T.G.; Park, B.; Jo, K.; Kim, C.S. New application for assessment of dry eye syndrome induced by particulate matter exposure. Ecotoxicol. Environ. Saf. 2020, 205, 111125. [Google Scholar] [CrossRef]
- Mu, N.; Wang, H.; Chen, D.; Wang, F.; Ji, L.; Zhang, C.; Li, M.; Lu, P. A novel rat model of dry eye induced by aerosol exposure of particulate matter. Investig. Ophthalmol. Vis. Sci. 2022, 63, 39. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Li, K.; Li, D.; Zhang, Y.; Liu, X.; Wu, K. Effects of fine particulate matter on the ocular surface: An in vitro and in vivo study. Biomed. Pharmacother. 2019, 117, 109177. [Google Scholar] [CrossRef]
- Song, F.; Chen, Z.; Lyu, D.; Gu, Z.; Lu, B.; Hao, S.; Xu, Y.; Jin, X.; Fu, Q.; Yao, K. Expression profiles of long noncoding RNAs in human corneal epithelial cells exposed to fine particulate matter. Chemosphere 2022, 287 Pt 1, 131955. [Google Scholar] [CrossRef]
- Brahma, I.; Ofili, O. Nucleation-accumulation mode trade-off in non-volatile particle emissions from a small non-road small diesel engine. Environ. Sci. Pollut. Res. Int. 2022, 29, 89449–89468. [Google Scholar] [CrossRef] [PubMed]
- Long, E.; Carlsten, C. Controlled human exposure to diesel exhaust: Results illuminate health effects of traffic-related air pollution and inform future directions. Part. Fibre Toxicol. 2022, 19, 11. [Google Scholar] [CrossRef]
- Kwon, M.; Jung, J.; Park, H.S.; Kim, N.H.; Lee, J.; Park, J.; Kim, Y.; Shin, S.; Lee, B.S.; Cheong, Y.H.; et al. Diesel exhaust particle exposure accelerates oxidative DNA damage and cytotoxicity in normal human bronchial epithelial cells through PD-L1. Environ. Pollut. 2022, 317, 120705. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Tang, L.; Shen, M.; Wang, Y.; Wei, Y.; Jeyalatha, V.; Chen, P.; Dong, F.; Wang, G.; Wu, S.; et al. Effects of diesel exhaust particles on the condition of mouse ocular surface. Ecotoxicol. Environ. Saf. 2018, 163, 585–593. [Google Scholar] [CrossRef]
- Lasagni Vitar, R.M.; Tau, J.; Janezic, N.S.; Tesone, A.I.; Hvozda Arana, A.G.; Reides, C.G.; Berra, A.; Ferreira, S.M.; Llesuy, S.F. Diesel exhaust particles (DEP) induce an early redox imbalance followed by an IL-6 mediated inflammatory response on human conjunctival epithelial cells. Exp. Eye Res. 2018, 171, 37–47. [Google Scholar] [CrossRef]
- Tau, J.; Novaes, P.; Matsuda, M.; Tasat, D.R.; Saldiva, P.H.; Berra, A. Diesel exhaust particles selectively induce both proinflammatory cytokines and mucin production in cornea and conjunctiva human cell lines. Investig. Ophthalmol. Vis. Sci. 2013, 54, 4759–4765. [Google Scholar] [CrossRef] [Green Version]
- Aik, J.; Chua, R.; Jamali, N.; Chee, E. The burden of acute conjunctivitis attributable to ambient particulate matter pollution in Singapore and its exacerbation during South-East Asian haze episodes. Sci. Total Environ. 2020, 740, 140129. [Google Scholar] [CrossRef]
- Chen, R.; Yang, J.; Zhang, C.; Li, B.; Bergmann, S.; Zeng, F.; Wang, H.; Wang, B. Global associations of air pollution and conjunctivitis diseases: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2019, 16, 3652. [Google Scholar] [CrossRef] [Green Version]
- Miyazaki, D.; Fukagawa, K.; Fukushima, A.; Fujishima, H.; Uchio, E.; Ebihara, N.; Shoji, J.; Takamura, E.; Namba, K.; Ohashi, Y.; et al. Air pollution significantly associated with severe ocular allergic inflammatory diseases. Sci. Rep. 2019, 9, 18205. [Google Scholar] [CrossRef] [Green Version]
- Min, K.B.; Min, J.Y. Association of ambient particulate matter exposure with the incidence of glaucoma in childhood. Am. J. Ophthalmol. 2020, 211, 176–182. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, J.W.; Kim, E.J.; Lee, M.Y.; Nam, C.W.; Chung, I.S. Spatial analysis between particulate matter and emergency room visits for conjunctivitis and keratitis. Ann. Occup. Environ. Med. 2018, 30, 41. [Google Scholar] [CrossRef]
- Cantó, A.; Olivar, T.; Romero, F.J.; Miranda, M. Nitrosative stress in retinal pathologies: Review. Antioxidants 2019, 8, 543. [Google Scholar] [CrossRef] [Green Version]
- Eroglu, E.; Charoensin, S.; Bischof, H.; Ramadani, J.; Gottschalk, B.; Depaoli, M.R.; Waldeck-Weiermair, M.; Graier, W.F.; Malli, R. Genetic biosensors for imaging nitric oxide in single cells. Free Radic. Biol. Med. 2018, 128, 50–58. [Google Scholar] [CrossRef]
- Tummanapalli, S.S.; Kuppusamy, R.; Yeo, J.H.; Kumar, N.; New, E.J.; Willcox, M.D.P. The role of nitric oxide in ocular surface physiology and pathophysiology. Ocul. Surf. 2021, 21, 37–51. [Google Scholar] [CrossRef]
- Mu, J.; Zeng, D.; Zeng, H. Effects of nitrogen dioxide exposure on the risk of eye and adnexa diseases among children in Shenzhen, China: An assessment using the generalized additive modeling approach. Int. J. Environ. Health Res. 2022, 32, 840–849. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, J.Y.; Kim, D.J.; Kim, M.; Chang, M.; Chuck, R.S.; Park, Y.C. Effect of nitric oxide on human corneal epithelial cell viability and corneal wound healing. Sci. Rep. 2017, 7, 8093. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.C.; Cheong, T.B.; Park, G.S.; Park, M.H.; Kwon, N.S.; Yoon, H.Y. The role of nitric oxide in ocular surface diseases. Adv. Exp. Med. Biol. 2002, 506 Pt A, 687–695. [Google Scholar] [CrossRef]
- Erdinest, N.; London, N.; Ovadia, H.; Levinger, N. Nitric oxide interaction with the eye. Vision 2021, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, J.S.; Ye, Y.Z.; Anderson, P.G.; Chen, J.; Accavitti, M.A.; Tarpey, M.M.; White, C.R. Extensive nitration of protein tyrosines in human atherosclerosis detected by immunohistochemistry. Biol. Chem. Hoppe Seyler 1994, 375, 81–88. [Google Scholar] [CrossRef]
- Ischiropoulos, H.; Zhu, L.; Chen, J.; Tsai, M.; Martin, J.C.; Smith, C.D.; Beckman, J.S. Peroxynitrite-mediated tyrosine nitration catalyzed by superoxide dismutase. Arch. Biochem. Biophys. 1992, 298, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.H.; Choi, Y.H.; Paik, H.J.; Wee, W.R.; Kim, M.K.; Kim, D.H. Potential importance of ozone in the association between outdoor air pollution and dry eye disease in South Korea. JAMA Ophthalmol. 2016, 134, 503–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novaes, H.M.; Gouveia, N.; de Medeiros, A. Perinatal mortality and traffic related air pollution. Rev. Bras. Ginecol. Obstet. 2010, 32, 471–475. [Google Scholar]
- Novaes, P.; Saldiva, P.H.; Matsuda, M.; Macchione, M.; Rangel, M.P.; Kara-José, N.; Berra, A. The effects of chronic exposure to traffic derived air pollution on the ocular surface. Environ. Res. 2010, 110, 372–374. [Google Scholar] [CrossRef]
- Malerbi, F.K.; Martins, L.C.; Saldiva, P.H.; Braga, A.L. Ambient levels of air pollution induce clinical worsening of blepharitis. Environ. Res. 2012, 112, 199–203. [Google Scholar] [CrossRef]
- Bernardes, T.F.; Bonfioli, A.A. Blepharitis. Semin. Ophthalmol. 2010, 25, 79–83. [Google Scholar] [CrossRef]
- Chang, K.H.; Hsu, P.Y.; Lin, C.J.; Lin, C.L.; Juo, S.H.; Liang, C.L. Traffic-related air pollutants increase the risk for age-related macular degeneration. J. Investig. Med. 2019, 67, 1076–1081. [Google Scholar] [CrossRef]
- Saxena, R.; Srivastava, S.; Trivedi, D.; Anand, E.; Joshi, S.; Gupta, S.K. Impact of environmental pollution on the eye. Acta Ophthalmol. Scand. 2003, 81, 491–494. [Google Scholar] [CrossRef]
- Yu, D.; Deng, Q.; Wang, J.; Chang, X.; Wang, S.; Yang, R.; Yu, J.; Yu, J. Air pollutants are associated with dry eye disease in urban ophthalmic outpatients: A prevalence study in China. J. Transl. Med. 2019, 17, 46. [Google Scholar] [CrossRef] [Green Version]
- Segui-Gomez, M. Driver air bag effectiveness by severity of the crash. Am. J. Public Health 2000, 90, 1575–1581. [Google Scholar] [CrossRef] [Green Version]
- Cummins, J.S.; Koval, K.J.; Cantu, R.V.; Spratt, K.F. Do seat belts and air bags reduce mortality and injury severity after car accidents? Am. J. Orthop. 2011, 40, E26–E29. [Google Scholar]
- Ulrich, D.; Noah, E.M.; Fuchs, P.; Pallua, N. Burn injuries caused by air bag deployment. Burns 2001, 27, 196–199. [Google Scholar] [CrossRef]
- Duma, S.M.; Jernigan, M.V. The effects of airbags on orbital fracture patterns in frontal automobile crashes. Ophthalmic Plast. Reconstr. Surg. 2003, 19, 107–111. [Google Scholar] [CrossRef]
- Stein, J.D.; Jaeger, E.A.; Jeffers, J.B. Air bags and ocular injuries. Trans. Am. Ophthalmol. Soc. 1999, 97, 59–82. [Google Scholar] [CrossRef] [Green Version]
- Ogun, O.A.; Ikyaa, S.Y.; Ogun, G.O. Rethinking airbag safety: Airbag injury causing bilateral blindness. Middle East Afr. J. Ophthalmol. 2014, 21, 196–199. [Google Scholar] [CrossRef]
- Fante, R.J.; Trobe, J.D. Images in clinical medicine. Bilateral corneal abrasions from airbag deployment. N. Engl. J. Med. 2014, 370, e40. [Google Scholar] [CrossRef] [Green Version]
- Fukagawa, K.; Tsubota, K.; Kimura, C. Corneal endothelial cell loss induced by air bags. Ophthalmology 1993, 12, 1819–1823. [Google Scholar] [CrossRef]
- Geggel, H.S.; Griggs, P.B.; Freeman, M.I. Irreversible bullous keratopathy after air bag trauma. CLAO J. 1996, 2, 148–150. [Google Scholar]
- Bansal, S.; Gunasekeran, D.V.; Ang, B.; Lee, J.; Khandelwal, R.; Sullivan, P.; Agrawal, R. Controversies in the pathophysiology and management of hyphema. Surv. Ophthalmol. 2016, 61, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Pearlman, J.A.; Au Eong, K.G.; Kuhn, F.; Pieramici, D.J. Airbags and eye injuries: Epidemiology, spectrum of injury, and analysis of risk factors. Surv. Ophthalmol. 2001, 46, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Brandt, M.T.; Haug, R.H. Traumatic hyphema: A comprehensive review. J. Oral Maxillofac. Surg. 2001, 59, 1462–1470. [Google Scholar] [CrossRef]
- Scarlett, A.; Gee, P. Corneal abrasion and alkali burn secondary to automobile air bag inflation. Emerg. Med. J. 2007, 24, 733–734. [Google Scholar] [CrossRef] [PubMed]
- Smally, A.J.; Binzer, A.; Dolin, S.; Viano, D. Alkaline chemical keratitis: Eye injury from airbags. Ann. Emerg. Med. 1992, 21, 1400–1402. [Google Scholar] [CrossRef]
- Barnes, S.S.; Wong, W., Jr.; Affeldt, J.C. A case of severe airbag related ocular alkali injury. Hawaii J. Med. Public Health 2012, 71, 229–231. [Google Scholar]
- Savastano, A.; Donati, M.C.; Rizzo, S. Retinal tear related to air bag deployment. J. Ophthalmol. 2016, 134, e155021. [Google Scholar] [CrossRef]
- DeLori, F.; Pomerantzeff, O.; Cox, M.S. Deformation of the globe under high-speed impact: Its relation to contusion injuries. Investig. Ophthalmol. 1969, 8, 290–301. [Google Scholar]
- Shirzadi, H.; Zohoor, H.; Naserkhaki, S. Biomechanical simulation of eye-airbag impacts during vehicle accidents. Proc. Inst. Mech. Eng. H 2018, 232, 699–707. [Google Scholar] [CrossRef]
- Maroni, M.; Fait, A.; Colosio, C. Risk assessment and management of occupational exposure to pesticides. Toxicol. Lett. 1999, 107, 145–153. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. World Food and Agriculture—Statistical Yearbook 2022; Food and Agriculture Organization of the United Nations: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Trask, C.; Khan, M.I.; Adebayo, O.; Boden, C.; Bath, B. Equity in whom gets studied: A systematic review examining geographical region, gender, commodity, and employment context in research of low back disorders in farmers. Agromedicine 2015, 20, 273–281. [Google Scholar] [CrossRef]
- Bish, M.; Oseland, E.; Bradley, K. Off-target pesticide movement: A review of our current understanding of drift due to inversions and secondary movement. Weed Technol. 2021, 35, 345–356. [Google Scholar] [CrossRef]
- Cech, R.; Zaller, J.G.; Lyssimachou, A.; Clausing, P.; Hertoge, K.; Linhart, C. Pesticide drift mitigation measures appear to reduce contamination of non-agricultural areas, but hazards to humans and the environment remain. Sci. Total Environ. 2022, 854, 158814. [Google Scholar] [CrossRef]
- Sanyal, S.; Das, P.; Law, S. Effect of chronic pesticide exposure on murine cornea: A histopathological, cytological and flow cytometric approach to study ocular damage by xenobiotics. Cell Biol. Toxicol. 2016, 32, 7–22. [Google Scholar] [CrossRef]
- Alozi, M.; Rawas-Qalaji, M. Treating organophosphates poisoning: Management challenges and potential solutions. Crit. Rev. Toxicol. 2020, 50, 764–779. [Google Scholar] [CrossRef]
- Coats, J.R. Mechanisms of toxic action and structure-activity relationships for organochlorine and synthetic pyrethroid insecticides. Environ. Health Perspect. 1990, 87, 255–262. [Google Scholar] [CrossRef]
- Jayara, J.R.; Megha, P.; Sreedev, P. Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdiscip. Toxicol. 2016, 9, 90–100. [Google Scholar] [CrossRef] [Green Version]
- Hou, C.; Wang, Z.; Li, X.; Bai, Y.; Chai, J.; Li, X.; Gao, J.; Xu, H. Study of modeling and optimization for predicting the acute toxicity of carbamate pesticides using the binding information with carrier protein. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 273, 121038. [Google Scholar] [CrossRef]
- Wesseling, C.; Aragón, A.; Castillo, L.; Corriols, M.; Chaverri, F.; de la Cruz, E.; Keifer, M.; Monge, P.; Partanen, T.J.; Ruepert, C.; et al. Hazardous pesticides in Central America. Int. J. Occup. Environ. Health 2001, 7, 287–294. [Google Scholar] [CrossRef]
- Mamane, A.; Baldi, I.; Tessier, J.F.; Raherison, C.; Bouvier, G. Occupational exposure to pesticides and respiratory health. Eur. Respir. Rev. 2015, 24, 306–319. [Google Scholar] [CrossRef] [Green Version]
- Aktar, M.W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clippinger, A.J.; Raabe, H.A.; Allen, D.G.; Choksi, N.Y.; van der Zalm, A.J.; Kleinstreuer, N.C.; Barroso, J.; Lowit, A.B. Human-relevant approaches to assess eye corrosion/irritation potential of agrochemical formulations. Cutan. Ocul. Toxicol. 2021, 40, 145–167. [Google Scholar] [CrossRef] [PubMed]
- Fareed, M.; Kesavachandran, C.N.; Pathak, M.K.; Bihari, V.; Kuddus, M.; Srivastava, A.K. Visual disturbances with cholinesterase depletion due to exposure of agricultural pesticides among farm workers. Toxicol. Environ. Chem. 2012, 94, 1601–1609. [Google Scholar] [CrossRef]
- Lu, J.L. Acute pesticide poisoning among cut-flower farmers. J. Environ. Health 2007, 70, 38–43. [Google Scholar] [PubMed]
- McKeag, D.; Maini, R.; Taylor, H.R. The ocular surface toxicity of paraquat. Br. J. Ophthalmol. 2002, 86, 350–351. [Google Scholar] [CrossRef] [Green Version]
- Joyce, M. Ocular damage caused by paraquat. Br. J. Ophthalmol. 1969, 53, 688–690. [Google Scholar] [CrossRef] [Green Version]
- Vlahos, K.; Goggin, M.; Coster, D. Paraquat causes chronic ocular surface toxicity. Aust. N. Z. J. Ophthalmol. 1993, 21, 187–190. [Google Scholar]
- Jian-Wei, L.; Xiu-Yun, L.; Ai-Jun, D. Effectiveness of heparin eye drops in paraquat-induced ocular injury. Cutan. Ocul. Toxicol. 2017, 36, 377–380. [Google Scholar] [CrossRef]
- Nirei, M.; Hayasaka, S.; Nagata, M.; Tamai, A.; Tawara, T. Ocular injury caused by Preeglox-L, a herbicide containing paraquat, diquat and surfactants. Jpn. J. Ophthalmol. 1993, 37, 43–46. [Google Scholar]
- Vale, A.; Lotti, M. Organophosphorus and carbamate insecticide poisoning. Handb. Clin. Neurol. 2015, 131, 149–168. [Google Scholar] [CrossRef]
- Ganie, S.Y.; Javaid, D.; Hajam, Y.A.; Reshi, M.S. Mechanisms and treatment strategies of organophosphate pesticide induced neurotoxicity in humans: A critical appraisal. Toxicology 2022, 472, 153181. [Google Scholar] [CrossRef]
- Amend, N.; Langgartner, J.; Siegert, M.; Kranawetvogl, T.; Koller, M.; John, H.; Pflügler, C.; Mögele-Schmid, C.; Worek, F.; Thiermann, H.; et al. A case report of cholinesterase inhibitor poisoning: Cholinesterase activities and analytical methods for diagnosis and clinical decision making. Arch. Toxicol. 2020, 94, 2239–2247. [Google Scholar] [CrossRef]
- Bradberry, S.M.; Proudfoot, A.T.; Vale, J.A. Glyphosate poisoning. Toxicol. Rev. 2004, 23, 159–167. [Google Scholar] [CrossRef]
- Anadón, A.; Martínez-Larrañaga, M.R.; Martínez, M.A.; Castellano, V.J.; Martínez, M.; Martin, M.T.; Nozal, M.J.; Bernal, J.L. Toxicokinetics of glyphosate and its metabolite aminmethyl phosphonic acid in rats. Toxicol. Lett. 2009, 190, 91–95. [Google Scholar] [CrossRef]
- Ma, P.; Wu, Y.; Zeng, Q.; Gan, Y.; Chen, J.; Ye, X.; Yang, X. Oxidative damage induced by chlorpyrifos in the hepatic and renal tissue of Kunming mice and the antioxidant role of vitamin E. Food Chem. Toxicol. 2013, 58, 177–183. [Google Scholar] [CrossRef]
- AlKahtane, A.A.; Ghanem, E.; Bungau, S.G.; Alarifi, S.; Daoud, A.; AlBasher, G.; Alkahtani, S.; Aleya, L.; Abdel-Daim, M.M. Carnosic acid alleviates chlorpyrifos-induced oxidative stress and inflammation in mice cerebral and ocular tissues. Environ. Sci. Pollut. Res. Int. 2020, 27, 11663–11670. [Google Scholar] [CrossRef]
- Nandi, N.K.; Vyas, A.; Akhtar, M.J.; Kumar, B. The growing concern of chlorpyrifos exposures on human and environmental health. Pestic. Biochem. Physiol. 2022, 185, 105138. [Google Scholar] [CrossRef]
- Aboubakr, M.; Elshafae, S.M.; Abdelhiee, E.Y.; Fadl, S.E.; Soliman, A.; Abdelkader, A.A.; Abdel-Daim, M.M.; Bayoumi, K.A.; Baty, R.S.; Elgendy, E. Antioxidant and anti-inflammatory potential of thymoquinone and lycopene mitigate the chlorpyrifos-induced toxic neuropathy. Pharmaceuticals 2021, 14, 940. [Google Scholar] [CrossRef]
- Hernández, A.F.; Lacasaña, M.; Gil, F.; Rodríguez-Barranco, M.; Pla, A.; López-Guarnido, O. Evaluation of pesticide-induced oxidative stress from a gene-environment interaction perspective. Toxicology 2013, 307, 95–102. [Google Scholar] [CrossRef]
- Banks, C.N.; Lein, P.J. A review of experimental evidence linking neurotoxic organophosphorus compounds and inflammation. Neurotoxicology 2012, 33, 575–584. [Google Scholar] [CrossRef] [Green Version]
- Goswami, D.G.; Kant, R.; Ammar, D.A.; Agarwal, C.; Gomez, J.; Agarwal, R.; Saba, L.M.; Fritz, K.S.; Tewari-Singh, N. Toxic consequences and oxidative protein carbonylation from chloropicrin exposure in human corneal epithelial cells. Toxicol. Lett. 2020, 322, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Medithi, S.; Kasa, Y.D.; Kankipati, V.R.; Kodali, V.; Jee, B.; Jonnalagadda, P.R. Impact of micronutrient supplementation on pesticide residual, acetylcholinesterase activity, and oxidative stress among farm children exposed to pesticides. Front. Public Health 2022, 10, 872125. [Google Scholar] [CrossRef] [PubMed]
- Samurkas, A.; Yao, L.; Hadiatullah, H.; Ma, R.; Xie, Y.; Sundarraj, R.; Zuilhof, H.Z. Ryanodine receptor as insecticide target. Curr. Pharm. Des. 2022, 28, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Roy, S. Flubendiamide induces transgenerational compound eye alterations in Drosophila melanogaster. Interdiscip. Toxicol. 2017, 10, 142–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarkar, S.; Roy, A.; Roy, S. Flubendiamide affects visual and locomotory activities of Drosophila melanogaster for three successive generations (P, F1 and F2). Invert. Neurosci. 2018, 18, 6. [Google Scholar] [CrossRef]
- Food Safety Commission of Japan. Flubenziamide (Pesticides). Food Saf. 2019, 7, 15–19. [Google Scholar] [CrossRef]
- Zakharov, S.; Csomor, J.; Urbanek, P.; Pelclova, D. Toxic epidermal necrolysis after exposure to dithiocarbamate fungicide Mancozeb. Basic Clin. Pharmacol Toxicol. 2016, 118, 87–91. [Google Scholar] [CrossRef]
- Colović, M.B.; Krstić, D.Z.; Lazarević-Pašti, T.D.; Bondžić, A.M.; Vasić, V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef] [Green Version]
- Matsukawa, T.; Yokoyama, K.; Itoh, H. Ocular irritation from product of pesticide degradation among workers in a seed warehouse. Ind. Health 2015, 53, 95–99. [Google Scholar] [CrossRef] [Green Version]
- Forrest, K.Y.; Cali, J.M. Epidemiology of lifetime work-related eye injuries in the U.S. population associated with one or more lost days of work. Ophthalmic Epidemiol. 2009, 16, 156–162. [Google Scholar] [CrossRef]
- Kyriakaki, E.D.; Symvoulakis, E.K.; Chlouverakis, G.; Detorakis, E.T. Causes, occupational risk and socio-economic determinants of eye injuries: A literature review. Med. Pharm. Rep. 2021, 94, 131–144. [Google Scholar] [CrossRef]
- Dua, H.S.; Ting, D.S.J.; Al Saadi, A.; Said, D.G. Chemical eye injury: Pathophysiology, assessment and management. Eye 2020, 34, 2001–2019. [Google Scholar] [CrossRef]
- Makwana, T.; Gupta, N.; Vashist, P. Ocular emergencies in the South Asia region. Community Eye Health 2019, 31, S1–S4. [Google Scholar]
- Peate, W.F. Work-related eye injuries and illnesses. Am. Fam. Physician 2007, 75, 1017–1022. [Google Scholar]
- McGwin, G.; Owsley, C. Incidence of emergency-department–treated eye injury in the United States. Arch. Ophthalmol. 2005, 123, 662–666. [Google Scholar] [CrossRef] [Green Version]
- Adriono, G.A.; Agustiawan, R.; Fibrian, K.C.; Ardiani, L.S.; Irawati, Y. Variations in clinical manifestations and outcomes of penetrating ocular injuries with intraocular foreign bodies: A case series. J. Surg. Case Rep. 2022, 2022, rjac198. [Google Scholar] [CrossRef]
- Khanam, S.; Agarwal, A.; Goel, R.; Rathie, N.; Raut, A.; Raghav, S.; Kumar, S.; Chhabra, M.; Singh, S.; Kumar, S. Clinical presentation and management strategies in intraorbital foreign bodies. Case Rep. Ophthalmol. Med. 2021, 2021, 6645952. [Google Scholar] [CrossRef]
- Hom, G.L.; Kalurm, A.; Iyer, A.; Singh, R.P. Ocular occupational injuries in the United States between 2011–2018. Occup. Med. 2022, 72, 255–259. [Google Scholar] [CrossRef]
- Awan, A.; Scott, J.A. Corneal injury from a fishing line: A new mechanism. Eye 2006, 20, 1084–1086. [Google Scholar] [CrossRef] [Green Version]
- Ono, T.; Takahashi, S.; Mori, Y.; Nejimar, R.; Iwasaki, T.; Kataoka, Y.; Miyai, T.; Miyata, K. 1Severe fishhook-related ocular injury: A case series. Trauma Case Rep. 2021, 37, 100574. [Google Scholar] [CrossRef]
- Choovuthayakorn, J.; Chavengsaksongkram, P.; Watanachai, N.; Chaidaroon, W. Penetrating eyelid and ocular fishhook-related injury. Case Rep. Ophthalmol. 2019, 24, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Purtskhvanidze, K.; Saeger, M.; Treumer, F.; Nölle, B.; Roider, J. Open globe and penetrating eyelid injuries from fish hooks. BMC Ophthalmol. 2019, 19, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haavisto, A.K.; Sahraravand, A.; Puska, P.; Leivo, T. Eye injuries caused by wooden projectiles in Finland. Wilderness Environ. Med. 2022, 33, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Fulcher, T.P.; McNab, A.A.; Sullivan, T.J. Clinical features and management of intraorbital foreign bodies. Ophthalmology 2002, 109, 494–500. [Google Scholar] [CrossRef]
- Al-Mujaini, A.; Al-Senawi, R.; Ganesh, A.; Al-Zuhaibi, S.; Al-Dhuhli, H. Intraorbital foreign body: Clinical presentation, radiological appearance and management. Sultan Qaboos Univ. Med. J. 2008, 8, 69–74. [Google Scholar]
- Hua, L.; Doll, T. A series of 3 cases of corneal abrasion with multiple etiologies. Optometry 2010, 81, 83–85. [Google Scholar] [CrossRef]
- You, Y.Y.; Shi, B.J.; Wang, X.Y.; Chen, J.; Wang, Z.R.; Wang, X.H.; Jiang, F.G. Intraorbital wooden foreign bodies: Case series and literature review. Int. J. Ophthalmol. 2021, 14, 1619–1627. [Google Scholar] [CrossRef]
- Pandit, K.; Sitaula, S.; Shrestha, G.B.; Joshi, S.N.; Chaudhary, M. Management of unusual missed diagnosis of a intra-orbital wooden foreign body: A case report and review of literature. Ann. Med. Surg. 2022, 79, 104017. [Google Scholar] [CrossRef]
- Li, J.; Zhou, L.P.; Jin, J.; Yuan, H.F. Clinical diagnosis and treatment of intraorbital wooden foreign bodies. Chin. J. Traumatol. 2016, 19, 322–325. [Google Scholar] [CrossRef]
- Ay, İ.E.; Demirezen, M.; Şenol, Y.; Til, A. Ocular health among industrial workers: A prevalence study of foreign body injury, refractive error, dry eye, pterygium and pingueculae. Med. Lav. 2022, 113, e2022044. [Google Scholar] [CrossRef]
- Liou, Y.H.; Chen, Y.J.; Chen, W.L.; Li, K.Y.; Chou, Y.; Huang, Y.C.; Wang, C.C.; Lai, C.H. Associations between biomarkers of metal exposure and dry eye metrics in shipyard welders: A cross-sectional study. Int. J. Environ. Res. Public Health 2022, 17, 2264. [Google Scholar] [CrossRef]
- Chen, Y.J.; Chen, Y.Y.; Lai, C.H. Clinical association between trace elements of tear and dry eye metrics. Sci. Rep. 2022, 12, 18052. [Google Scholar] [CrossRef]
- Bouirig, K.; Cherkaoui, O. Iron deposition from a retained intraocular foreign body. N. Engl. J. Med. 2022, 387, e49. [Google Scholar] [CrossRef]
- Khanduja, S.; Khurana, A.; Sachdeva, S.; Rathi, A.; Khurana, A.K. Tractor nail as impacted foreign body: Rare case scenario. Int. Ophthalmol. 2013, 33, 291–293. [Google Scholar] [CrossRef]
- Irving Enrique, C.S.; Dhariana, A.R.; Vidal, S.V.; Carlos Felipe, P.H.; Lorena, W.G.; Gerardo, G.A. Conservative management of penetrating ocular trauma caused by a nail gun. Am. J. Ophthalmol. Case Rep. 2018, 11, 115–118. [Google Scholar] [CrossRef]
- Burger, B.M.; Kelty, P.J.; Bowie, E.M. Ocular nail gun injuries: Epidemiology and visual outcomes. J. Trauma 2009, 67, 1320–1322. [Google Scholar] [CrossRef]
- Elahi, S.; Saad, A.; Gatinel, D. Descemet membrane endothelial keratoplasty for corneal decompensation due to migrating metallic intracorneal foreign bodies in an aphakic eye following a 39-year-old blast injury: A case report. Am. J. Ophthalmol. Case Rep. 2021, 23, 101162. [Google Scholar] [CrossRef]
- Al-Dwairi, R.; Msallam, M. Unilateral ocular siderosis bulbi due to missed metallic intraocular foreign body masquerading as anisocoria of neurological origin: A case report. Am. J. Case. Rep. 2021, 22, e930504. [Google Scholar] [CrossRef]
- Doctor, M.B.; Parameswarappa, D.C.; Vaddavalli, P.K.; Rani, P.K. Intralenticular copper foreign body. BMJ Case Rep. 2020, 13, e240757. [Google Scholar] [CrossRef]
- Ramakrishnan, T.; Constantinou, M.; Jhanji, V.; Vajpayee, R.B. Corneal metallic foreign body injuries due to suboptimal ocular protection. Arch. Environ. Occup. Health 2012, 67, 48–50. [Google Scholar] [CrossRef]
- Said, D.; Harminder, D. Chemical burns acid or alkali, what’s the difference? Eye 2020, 34, 1299–1300. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghadeer, H.; Al Amry, M.; Aldihan, K.A.; Alobaidan, O.S.; AlQahtani, G.M.S.; Khandekar, R. Demographic, clinical profile and management outcomes of ocular chemical injuries in Saudi children. Clin. Ophthalmol. 2022, 16, 3247–3255. [Google Scholar] [CrossRef] [PubMed]
- Bizrah, M.; Yusuf, A.; Ahmad, S. An update on chemical eye burns. Eye 2019, 33, 1362–1377. [Google Scholar] [CrossRef] [PubMed]
- Tuft, S.J.; Shortt, A.J. Surgical rehabilitation following severe ocular burns. Eye 2009, 23, 1966–1971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamboj, A.; Spiller, H.A.; Casavant, M.J.; Kistamgari, S.; Chounthirath, T.; Smith, G.A. Household cleaning product-related ocular exposures reported to the United States poison control centres. Eye 2020, 34, 1631–1639. [Google Scholar] [CrossRef]
- Slaughter, R.J.; Watts, M.; Vale, J.A.; Grieve, J.R.; Schep, L.J. The clinical toxicology of sodium hypochlorite. Clin. Toxicol. 2019, 57, 303–311. [Google Scholar] [CrossRef]
- Tredici, C.; Fasciani, R.; Villano, A.; Gambini, G.; Caporossi, A. Efficacy of eye drops containing crosslinked hyaluronic acid and CoQ10 in restoring ocular health exposed to chlorinated water. Eur. J. Ophthalmol. 2020, 30, 430–438. [Google Scholar] [CrossRef]
- Blackburn, J.; Levitan, E.B.; MacLennan, P.A.; Owsley, C.; McGwin, G., Jr. The epidemiology of chemical eye injuries. Curr. Eye Res. 2012, 37, 787–793. [Google Scholar] [CrossRef]
- Bajraktarova-Valjakova, E.; Korunoska-Stevkovska, V.; Georgieva, S.; Ivanovski, K.; Bajraktarova-Misevska, C.; Mijoska, A.; Grozdanov, A. Hydrofluoric acid: Burns and systemic toxicity, protective measures, immediate and hospital medical treatment. Open Access Maced. J. Med. Sci. 2018, 6, 2257–2269. [Google Scholar] [CrossRef] [Green Version]
- Atley, K.; Ridyard, E. Treatment of hydrofluoric acid exposure to the eye. Int. J. Ophthalmol. 2015, 8, 157–161. [Google Scholar] [CrossRef]
- Lee, J.; Jun, J.H. Ocular chemical burn associated with gel type alcohol-based hand sanitizer: A case report. Medicine 2021, 100, e27292. [Google Scholar] [CrossRef]
- Oh, J.Y.; Yu, J.M.; Ko, J.H. Analysis of ethanol effects on corneal epithelium. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3852–3856. [Google Scholar] [CrossRef] [Green Version]
- Claassen, K.; Rodil Dos Anjos, D.; Broding, H.C. Current status of emergency treatment of chemical eye burns in workplaces. Int. J. Ophthalmol. 2021, 14, 306–309. [Google Scholar] [CrossRef]
- Lipscomb, H.J. Effectiveness of interventions to prevent work-related eye injuries. Am. J. Prev. Med. 2000, 18, 27–32. [Google Scholar] [CrossRef]
- Balkhyour, M.A.; Ahmad, I.; Rehan, M. Assessment of personal protective equipment use and occupational exposures in small industries in Jeddah: Health implications for workers. Saudi J. Biol. Sci. 2019, 26, 653–659. [Google Scholar] [CrossRef]
- Dain, S.J.; Huang, R.; Tiao, A.; Chou, B.R. When is protection from impact needed for the face as well as the eyes in occupational environments? Clin. Exp. Optom. 2018, 101, 392–396. [Google Scholar] [CrossRef]
- Abu, E.K.; Ocansey, S.; Gyamfi, J.A.; Ntodie, M.; Morny, E.K. Epidemiology and visual outcomes of ocular injuries in a low resource country. Afr. Health Sci. 2020, 20, 779–788. [Google Scholar] [CrossRef]
- Ahmed, F.; House, R.J.; Feldman, B.H. Corneal abrasions and corneal foreign bodies. Prim. Care 2015, 42, 363–375. [Google Scholar] [CrossRef]
- Monaghan, P.F.; Bryant, C.A.; McDermott, R.J.; Forst, L.S.; Luque, J.S.; Contreras, R.B. Adoption of safety eyewear among citrus harvesters in rural Florida. J. Immigr. Minor. Health 2012, 14, 460–466. [Google Scholar] [CrossRef]
- Sun, F.; Zhou, Y.; Dong, L.; Qin, H. Relationship between the use and type of eye protection and work-related corneal and conjunctival foreign body injuries. Inj. Prev. 2021, 27, 521–526. [Google Scholar] [CrossRef]
- Fann, N.; Nolte, C.G.; Dolwick, P.; Spero, T.L.; Brown, A.C.; Phillips, S.; Anenberg, S. The geographic distribution and economic value of climate change-related ozone health impacts in the United States in 2030. J. Air Waste Manag. Assoc. 2015, 65, 570–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, M.; Goldberg, R.; Hogrefe, C.; Kinney, P.L.; Knowlton, K.; Lynn, B.; Rosenthal, J.; Rosenzweig, C.; Patz, J.A. Climate change, ambient ozone, and health in 50 US cities. Clim. Chang. 2007, 82, 61–76. [Google Scholar] [CrossRef]
- Neta, G.; Pan, W.; Ebi, K.; Buss, D.F.; Castranio, T.; Lowe, R.; Ryan, S.J.; Stewart-Ibarra, A.M.; Hapairai, L.K.; Sehgal, M. Advancing climate change health adaptation through implementation science. Lancet Planet Health 2022, 6, e909–e918. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D.; Askari, S.; Gibson, S.; Bloem, M.W.; Kraemer, K. The potential impact of climate change on the micronutrient-rich food supply. Adv. Nutr. 2022, 13, 80–100. [Google Scholar] [CrossRef] [PubMed]
- Brönnimann, S.; Martín, J.-C.; Eugene, R.; Andreas, M.F.; Olaf, M.; Guang, Z.; Hideharu, A.; Yousuke, Y. Tropical circulation and precipitation response to ozone depletion and recovery. Environ. Res. Lett. 2017, 12, 064011. [Google Scholar] [CrossRef]
- Wu, Y.; Polvani, L.M. Recent trends in extreme precipitation and temperature over southeastern South America: The dominant role of stratospheric ozone depletion in the CESM Large Ensemble. J. Clim. 2017, 30, 6433–6441. [Google Scholar] [CrossRef]
- Ivy, D.J.; Solomon, S.; Calvo, N.; Thompson, D.W.J. Observed connections of Arctic stratospheric ozone extremes to Northern Hemisphere surface climate. Environ. Res. Lett. 2017, 12, 024004. [Google Scholar] [CrossRef]
- Barnes, P.W.; Robson, T.M.; Neale, P.J.; Williamson, C.E.; Zepp, R.G.; Madronich, S.; Wilson, S.R.; Andrady, A.L.; Heikkilä, A.M.; Bernhard, G.H. Environmental effects of stratospheric ozone depletion, UV radiation, and interactions with climate change: UNEP Environmental Effects Assessment Panel, Update 2021. Photochem. Photobiol. Sci. 2022, 21, 275–301. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2022: Impacts, Adaptation, and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Pörtner, H.-O., Roberts, D.C., Tignor, M., Poloczanska, E.S., Mintenbeck, K., Alegría, A., Craig, M., Langsdorf, S., Löschke, S., Möller, V., et al., Eds.; Cambridge University Press: Cambridge, UK, 2022. [Google Scholar] [CrossRef]
- Pandipati, S.; Abel, D.E. Anticipated impacts of climate change on women’s health: A background primer. Int. J. Gynaecol. Obstet. 2023, 160, 394–399. [Google Scholar] [CrossRef]
- El Hamichi, S.; Gold, A.; Murray, T.G.; Graversen, V.K. Pandemics, climate change, and the eye. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 2597–2601. [Google Scholar] [CrossRef]
- Echevarría-Lucas, L.; Senciales-González, J.M.; Medialdea-Hurtado, M.E.; Rodrigo-Comino, J. Impact of climate change on eye diseases and associated economical costs. Int. J. Environ. Res. Public Health 2021, 18, 7197. [Google Scholar] [CrossRef]
- Laporta, J. ADSA Foundation Scholar Award: Early-life exposure to hyperthermia: Productive and physiological outcomes, costs, and opportunities. J. Dairy Sci. 2021, 104, 11337–11347. [Google Scholar] [CrossRef]
- The Lancet. Global heating: An urgent call for action to protect health. Lancet 2022, 400, 1557. [Google Scholar] [CrossRef]
- Liu, H.; Tong, M.; Guo, F.; Nie, Q.; Li, J.; Li, P.; Zhu, T.; Xue, T. Deaths attributable to anomalous temperature: A generalizable metric for the health impact of global warming. Environ. Int. 2022, 169, 107520. [Google Scholar] [CrossRef]
- Johnson, G.J. The environment and the eye. Eye 2004, 18, 1235–1250. [Google Scholar] [CrossRef] [Green Version]
- Langley, R.K.; Mortimer, C.B.; McCulloch, C. The experimental production of cataracts by exposure to heat and light. Arch. Ophthalmol. 1960, 63, 473–488. [Google Scholar] [CrossRef]
- Kessel, L.; Johnson, L.; Arvidsson, H.; Larsen, M. The relationship between body and ambient temperature and corneal temperature. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6593–6597. [Google Scholar] [CrossRef]
- Walkden, A.; Fullwood, C.; Tan, S.Z.; Au, L.; Armstrong, M.; Brahma, A.K.; Chidambaram, J.D.; Carley, F. Association between season, temperature and causative organism in microbial keratitis in the UK. Cornea 2018, 37, 1555–1560. [Google Scholar] [CrossRef]
- Pupić-Bakrač, A.; Pupić-Bakrač, J.; Škara Kolega, M.; Beck, R. Human ophthalmomyiasis caused by Oestrus ovis–first report from Croatia and review on cases from Mediterranean countries. Parasitol. Res. 2020, 119, 783–793. [Google Scholar] [CrossRef]
- Mungroo, M.R.; Khan, N.A.; Maciver, S.; Siddiqui, R. Opportunistic free-living amoebal pathogens. Pathog. Glob. Health 2022, 116, 70–84. [Google Scholar] [CrossRef]
- Tsai, M.J.; Hsu, Y.L.; Wu, K.Y.; Yang, R.-C.; Chen, Y.-J.; Yu, H.-S.; Kuo, P.-L. Heat effect induces production of inflammatory cytokines through heat shock protein 90 pathway in cornea cells. Curr. Eye Res. 2013, 38, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghadyan, A.A.; Cotlier, E. Rise in lens temperature on exposure to sunlight or high ambient temperature. Br. J. Ophthalmol. 1986, 70, 421–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berg, E.J.; Ying, G.S.; Maguire, M.G.; Sheffield, P.E.; Szczotka-Flynn, L.B.; Asbell, P.A.; Shen, J.F.; DREAM Study Research Group. Climatic and environmental correlates of dry eye disease severity: A report from the Dry Eye Assessment and Management (DREAM) Study. Transl. Vis. Sci. Technol. 2020, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; An, X.; Sun, Z.; Li, Y.; Hou, Q. Identification of health effects of complex air pollution in China. Int. J. Environ. Res. Public Health 2022, 19, 12652. [Google Scholar] [CrossRef] [PubMed]
- Luschkova, D.; Traidl-Hoffmann, C.; Ludwig, A. Climate change and allergies. Allergo J. Int. 2022, 31, 114–120. [Google Scholar] [CrossRef]
- Reis, L.A.; Drouet, L.; Tavoni, M. Internalising health-economic impacts of air pollution into climate policy: A global modelling study. Lancet Planet Health 2022, 6, e40–e48. [Google Scholar] [CrossRef]
- Klopfer, J. Effects of environmental air pollution on the eye. J. Am. Optom. Assoc. 1989, 60, 773–778. [Google Scholar]
- Gupta, S.K.; Gupta, S.C.; Agarwal, R.; Sushma, S.; Agrawal, S.S.; Saxena, R. A multicentric case-control study on the impact of air pollution on eyes in a metropolitan city of India. Indian J. Occup. Environ. Med. 2007, 11, 37–40. [Google Scholar] [CrossRef] [Green Version]
- Hine, C.H.; Hogan, M.J.; McEwen, W.K.; Meyers, F.H.; Mettier, S.R.; Boyer, H.K. Eye irritation from air pollution. J. Air Pollut. Control Assoc. 1960, 10, 17–20. [Google Scholar] [CrossRef] [Green Version]
- Altshuller, A.P. Eye irritation as an effect of photochemical air pollution. J. Air Pollut. Control Assoc. 1977, 27, 1125–1126. [Google Scholar] [CrossRef] [Green Version]
- Jing, D.; Jiang, X.; Zhou, P.; Ren, X.; Su, J.; Hao, R.; Zhang, M.; Wan, Y.; Li, X. Evidence of air pollution-related ocular signs and altered inflammatory cytokine profile of the ocular surface in Beijing. Sci. Rep. 2022, 12, 18359. [Google Scholar] [CrossRef]
- Keramatnejad, M.; DeWolf, C. Impact of Pollutant Ozone on the Biophysical Properties of Tear Film Lipid Layer Model Membranes. Membranes 2023, 13, 165. [Google Scholar] [CrossRef]
- Kim, Y.; Paik, H.J.; Kim, M.K.; Choi, Y.H.; Kim, D.H. Short-term effects of ground-level ozone in patients with dry eye disease: A prospective clinical study. Cornea 2019, 38, 1483–1488. [Google Scholar] [CrossRef]
- McKenzie, R.L.; Liley, J.B.; Björn, L.O. UV radiation: Balancing risks and benefits. Photochem. Photobiol. 2009, 85, 88–98. [Google Scholar] [CrossRef]
- Roberts, J.E. Ocular phototoxicity. J. Photochem. Photobiol. B 2001, 64, 136–143. [Google Scholar] [CrossRef]
- Hu, D.N.; Simon, J.D.; Sarna, T. Role of ocular melanin in ophthalmic physiology and pathology. Photochem. Photobiol. 2008, 84, 639–644. [Google Scholar] [CrossRef]
- Lou, M.F. Glutathione and Glutaredoxin in redox regulation and cell signaling of the lens. Antioxidants 2022, 11, 1973. [Google Scholar] [CrossRef]
- Benedict, G.B. Theory of transparency of the eye. Appl. Opt. 1971, 10, 459–473. [Google Scholar] [CrossRef]
- Hiller, R.; Giacometti, L.; Yuen, K. Sunlight and cataract: An epidemiologic investigation. Am. J. Epidemiol. 1977, 105, 450–459. [Google Scholar] [CrossRef]
- Brilliant, L.B.; Grasset, N.C.; Pokhrel, R.P.; Kolstad, A.; Lepkowski, J.M.; Brilliant, G.E.; Hawks, W.M.; Pararajasegaram, R. Associations among cataract prevalence, sunlight hours, and altitude in the Himalayas. Am. J. Epidemiol. 1983, 118, 250–264. [Google Scholar] [CrossRef]
- Kamari, F.; Hallaj, S.; Dorosti, F.; Alinezhad, F.; Taleschian-Tabrizi, N.; Farhadi, F.; Aslani, H. Phototoxicity of environmental radiations in human lens: Revisiting the pathogenesis of UV-induced cataract. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 2065–2077. [Google Scholar] [CrossRef] [PubMed]
- Andley, U.P. The lens epithelium: Focus on the expression and function of the α-crystalline chaperones. Int. J. Biochem. Cell Biol. 2008, 40, 317–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willmann, G. Ultraviolet keratitis: From the pathophysiological basis to prevention and clinical management. High Alt. Med. Biol. 2015, 16, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Offord, E.A.; Sharif, N.A.; Macé, K.; Tromvoukis, Y.; Spillare, E.A.; Avanti, O.; Howe, W.E.; Pfeifer, A.M. Immortalized human corneal epithelial cells for ocular toxicity and inflammation studies. Investig. Ophthalmol. Vis. Sci. 1999, 40, 1091–1101. [Google Scholar]
- Taşkıran Kandeğer, B. Mass photokeratitis in coronary angiography medical staff following exposure to unprotected ultraviolet light. J. Fr. Ophtalmol. 2021, 44, e317–e318. [Google Scholar] [CrossRef]
- Volatier, T.; Schumacher, B.; Cursiefen, C.; Notara, M. UV Protection in the cornea: Failure and rescue. Biology 2022, 11, 278. [Google Scholar] [CrossRef]
- Izadi, M.; Jonaidi-Jafari, N.; Pourazizi, M.; Alemzadeh-Ansari, M.H.; Hoseinpourfard, M.J. Photokeratitis induced by ultraviolet radiation in travelers: A major health problem. J. Postgrad. Med. 2018, 64, 40–46. [Google Scholar] [CrossRef]
- Lucas, R.M. An epidemiological perspective of ultraviolet exposure--public health concerns. Eye Contact Lens 2011, 37, 168–175. [Google Scholar] [CrossRef]
- Gichuhi, S.; Ohnuma, S.; Sagoo, M.S.; Burton, M.J. Pathophysiology of ocular surface squamous neoplasia. Exp. Eye Res. 2014, 129, 172–182. [Google Scholar] [CrossRef] [Green Version]
- Haworth, K.M.; Chandler, H.L. Seasonal effect on ocular sun exposure and conjunctival UV autofluorescence. Optom. Vis. Sci. 2017, 94, 219–228. [Google Scholar] [CrossRef] [Green Version]
- Sherwin, J.C.; Hewitt, A.W.; Kearns, L.S.; Griffiths, L.R.; Mackey, D.A.; Coroneo, M.T. The association between pterygium and conjunctival ultraviolet autofluorescence: The Norfolk Island Eye Study. Acta Ophthalmol. 2013, 91, 363–370. [Google Scholar] [CrossRef]
- Zhou, W.P.; Zhu, Y.F.; Zhang, B.; Qiu, W.Y.; Yao, Y.F. The role of ultraviolet radiation in the pathogenesis of pterygia (Review). Mol. Med. Rep. 2016, 14, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Rivolta, C.; Royer-Bertrand, B.; Rimoldi, D.; Schalenbourg, A.; Zografos, L.; Leyvraz, S.; Moulin, A. UV light signature in conjunctival melanoma; not only skin should be protected from solar radiation. J. Hum. Genet. 2016, 61, 361–362. [Google Scholar] [CrossRef] [Green Version]
- Bais, A.F.; Lucas, R.M.; Bornman, J.F.; Williamson, C.E.; Sulzberger, B.; Austin, A.T.; Wilson, S.R.; Andrady, A.L.; Bernhard, G.; McKenzie, R.L.; et al. Environmental effects of ozone depletion, UV radiation and interactions with climate change: UNEP Environmental Effects Assessment Panel, update 2017. Photochem. Photobiol. Sci. 2018, 17, 127–179. [Google Scholar] [CrossRef]
- Paulson, C.; Thomas, S.C.; Gonzalez, O.; Taylor, S.; Swiston, C.; Herrick, J.S.; McCoy, L.; Curtin, K.; Chaya, C.J.; Stagg, B.C.; et al. Exfoliation yndrome in Baja Verapaz Guatemala: A cross-sectional study and review of the literature. J. Clin. Med. 2022, 11, 1795. [Google Scholar] [CrossRef]
- Sureshkumar, I.; Gunalan, V.; Nareshkumar, R.N.; Sripriya, K.; Ronnie, G.; Sharada, R.; Asokan, R. Evaluating the impact of ocular UV exposure for the development for pseudoexfoliation syndrome in a South Indian population. Clin. Exp. Optom. 2022, 14, 1–7. [Google Scholar] [CrossRef]
- Schmidt, R.E.; Zuclich, J.A. Retinal lesions due to ultraviolet laser exposure. Investig. Ophthalmol. Vis. Sci. 1980, 19, 1166–1175. [Google Scholar]
- Glickman, R.D. Ultraviolet phototoxicity to the retina. Eye Contact Lens 2011, 37, 196–205. [Google Scholar] [CrossRef]
- Boulton, M.; Rózanowska, M.; Rózanowski, B. Retinal photodamage. J. Photochem. Photobiol. B 2001, 64, 144–161. [Google Scholar] [CrossRef]
- Alven, A.; Lema, C.; Redfern, R.L. Impact of low humidity on damage-associated molecular patterns at the ocular surface during dry eye disease. Optom. Vis. Sci. 2021, 98, 1231–1238. [Google Scholar] [CrossRef]
- Di Carlo, E.; Augustin, A.J. Prevention of the onset of age-related macular degeneration. J. Clin. Med. 2021, 10, 3297. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, H.; Gali, H.E.; Bu, J.J.; Sella, R.; Afshari, N.A. Ultraviolet light exposure and its penetrance through the eye in a porcine model. Int. J. Ophthalmol. 2023, 16, 172–177. [Google Scholar] [CrossRef] [PubMed]

| Type of Burn | Chemical Causes | Where Found |
|---|---|---|
| Alkali | Calcium carbonate, magnesium carbonate | Lime |
| Alkali | Calcium hydroxide | Plaster, mortar, cement |
| Alkali | Sodium hydroxide | Drain cleaner |
| Alkali | Potassium hydroxide | Caustic potash, liquid fertilizer, soft soaps |
| Alkali | Magnesium hydroxide | Fireworks, sparklers |
| Alkali | Ammonium hydroxide | Cleaning agents, fertilizers, window cleaner |
| Alkali | Sodium tripolyphosphate | Dish detergent, kitchen and bathroom cleaners |
| Acidic | Hydrofluoric acid | Glass polisher, rust remover, industrial cleaners |
| Acidic | Hydrochloric acid | Food and leather-processing compounds, swimming pools |
| Acidic | Sulfuric acid | Toilet cleaner, battery fluid |
| Acidic | Sodium hypochlorite, calcium hypochlorite | Bleach, pool cleaners |
| Acidic | Acetic acid | Vinegar |
| Alcohol | Ethanol | Hand sanitizer |
| Alcohol | Methanol | Industrial solvents, pesticides |
| Alcohol | Isopropanol | Antifreeze, disinfectants, antiseptics |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rauchman, S.H.; Locke, B.; Albert, J.; De Leon, J.; Peltier, M.R.; Reiss, A.B. Toxic External Exposure Leading to Ocular Surface Injury. Vision 2023, 7, 32. https://doi.org/10.3390/vision7020032
Rauchman SH, Locke B, Albert J, De Leon J, Peltier MR, Reiss AB. Toxic External Exposure Leading to Ocular Surface Injury. Vision. 2023; 7(2):32. https://doi.org/10.3390/vision7020032
Chicago/Turabian StyleRauchman, Steven H., Brandon Locke, Jacqueline Albert, Joshua De Leon, Morgan R. Peltier, and Allison B. Reiss. 2023. "Toxic External Exposure Leading to Ocular Surface Injury" Vision 7, no. 2: 32. https://doi.org/10.3390/vision7020032