Allometric Growth of the Enigmatic Deep-Sea Megamouth Shark Megachasma pelagios Taylor, Compagno, and Struhsaker, 1983 (Lamniformes, Megachasmidae)
Abstract
:1. Introduction
2. Materials and Methods
3. Results
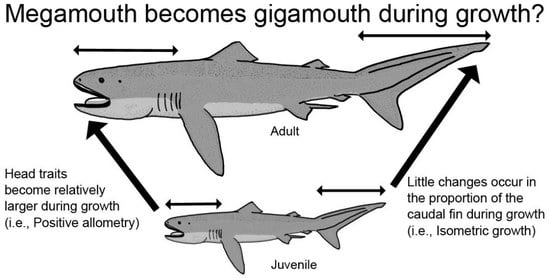
3.1. Head Measurements
3.2. Body Measurements
3.3. Dorsal Fin Measurements
3.4. Pectoral Fin Measurements
3.5. Caudal Fin Measurements
3.6. Comparisons with Other Lamniform Shark Species
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taylor, L.R.; Compagno, L.J.V.; Struhsaker, P.J. Megamouth—A new species, genus, and family of lamnoid shark (Megachasma pelagios, family Megachasmidae) from the Hawaiian Islands. Proc. Calif. Acad. Sci. 1983, 43, 87–110. [Google Scholar]
- Maisey, J.G. Relationships of the megamouth shark, Megachasma. Copeia 1985, 1, 228–231. [Google Scholar] [CrossRef]
- Martin, A.P.; Naylor, G.J. Independent origins of filter-feeding in megamouth and basking sharks (order Lamniformes) inferred from phylogenetic analysis of cytochrome b gene sequences. In Biology of the Magamouth Shark; Yano, K., Morrissey, J.F., Yabumoto, Y., Nakaya, K., Eds.; Tokai University Press: Tokyo, Japan, 1997; pp. 39–50. [Google Scholar]
- Naylor, G.J.P.; Martin, A.P.; Mattison, E.G.; Brown, W.M. Interrelationships of lamniform sharks: Testing phylogenetic hypotheses with sequence data. In Molecular Systematics of Fishes; Kocher, T.D., Stepien, C.A., Eds.; Academic Press: New York, NY, USA, 1997; pp. 199–218. [Google Scholar]
- Compagno, L.J.V. Sharks of the World: An Annotated and Illustrated Catalogue of Shark Species Known to Date: Bullhead, Mackerel and Carpet Sharks (Heterodontiformes, Lamniformes and Orectolobiformes); FAO Species Identification Guides for Fishery Purposes; Food and Agriculture Organization of the United Nations: Rome, Italy, 2001; Volume 2, p. 269. [Google Scholar]
- Mitchell, M.G.; Ciampaglio, C.N.; Jacquemin, S.J. Convergent evolution in tooth morphology of filter-feeding lamniform sharks. Southeast. Geol. 2018, 53, 63–80. [Google Scholar]
- Yu, C.-J.; Joung, S.-J.; Hsu, H.-H.; Lin, C.-Y.; Hsieh, T.-C.; Liu, K.-M.; Yamaguchi, A. Spatial–Temporal Distribution of Megamouth Shark, Megachasma pelagios, inferred from over 250 Individuals Recorded in the Three Oceans. Animals 2021, 11, 2947. [Google Scholar] [CrossRef] [PubMed]
- Nakaya, K. Biology of the megamouth shark, Megachasma pelagios (Lamniformes: Megachasmidae). In Proceedings of the International Symposium, into the Unknown, Researching Mysterious Deep-Sea Animals, Okinawa, Japan, 23–24 February 2007; pp. 69–83. [Google Scholar]
- Ebert, D.A.; Dando, M. On Board Guide for the Identification of Pelagic Sharks and Rays of the Western Indian Ocean; SmartFish Programme; Food and Agriculture Organization of the United Nations; Rome & Indian Ocean Commission: Port Louis, Mauritius, 2014; p. 109. [Google Scholar]
- Kyne, P.M.; Liu, K.M.; Simpfendorfer, C. Megachasma Pelagios. In The IUCN Red List of Threatened Species; IUCN Global Species Programme Red List Unit: Cambridge, UK, 2019. [Google Scholar]
- Watanabe, Y.Y.; Papastamatiou, Y.P. Distribution, body size and biology of the megamouth shark Megachasma pelagios. J. Fish Biol. 2019, 95, 992–998. [Google Scholar] [CrossRef]
- Liu, S.Y.V.; Joung, S.J.; Yu, C.-J.; Hsu, H.-H.; Tsai, W.-P.; Liu, K.M. Genetic diversity and connectivity of the megamouth shark (Megachasma pelagios). PeerJ 2018, 6, e4432. [Google Scholar] [CrossRef] [Green Version]
- Weigmann, S. Annotated checklist of the living sharks, batoids and chimaeras (Chondrichthyes) of the world, with a focus on biogeographical diversity. J. Fish. Biol. 2016, 88, 837–1037. [Google Scholar] [CrossRef]
- Irschick, D.J.; Hammerschlag, N. Morphological scaling of body form in four shark species differing in ecology and life history. Biol. J. Linn. Soc. 2015, 114, 126–135. [Google Scholar] [CrossRef] [Green Version]
- Ahnelt, H.; Sauberer, M.; Ramler, D.; Koch, L.; Pogoreutz, C. Negative allometric growth during ontogeny in the large pelagic filter-feeding basking shark. Zoomorphology 2020, 139, 71–83. [Google Scholar] [CrossRef] [Green Version]
- Sternes, P.C.; Higham, T.E. Hammer it out: Shifts in habitat are associated with changes in fin and body shape in the scalloped hammerhead (Sphyrna lewini). Biol. J. Linn. Soc. 2022, 136, 201–212. [Google Scholar] [CrossRef]
- Rohner, C.A.; Richardson, A.J.; Marshall, A.D.; Weeks, S.J.; Pierce, S.J. How large is the world’s largest fish? Measuring whale sharks Rhincodon typus with laser photogrammetry. J. Fish Biol. 2011, 78, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Reiss, K.L.; Bonnan, M.F. Ontogenetic scaling of caudal fin shape in Squalus acanthias (Chondrichthyes, Elasmobranchii): A geometric morphometric analysis with implications for caudal fin functional morphology. Anat. Rec. 2010, 293, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Fu, A.L.; Hammerschlag, N.; Lauder, G.V.; Wilga, C.D.; Kuo, C.-Y.; Irschick, D.J. Ontogeny of head and caudal fin shape of an apex marine predator: The tiger shark (Galeocerdo cuvier). J. Morphol. 2016, 277, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Irschick, D.J.; Fu, A.; Lauder, G.; Wilga, C.; Kuo, C.-Y.; Hammerschlag, N. A comparative morphological analysis of body and fin shape for eight shark species. Biol. J. Linn. Soc. 2017, 122, 589–604. [Google Scholar] [CrossRef] [Green Version]
- Cooper, J.A.; Pimiento, C.; Ferrón, H.G.; Benton, M.J. Body dimensions of the extinct giant shark Otodus megalodon: A 2D reconstruction. Sci. Rep. 2020, 10, 14596. [Google Scholar] [CrossRef]
- Lingham-Soliar, T. Caudal fin allometry in the white shark Carcharodon carcharias: Implications for locomotory performance and ecology. Naturwissen 2005, 92, 231–236. [Google Scholar] [CrossRef]
- Amorim, A.F.; Arfeli, C.A.; Castro, J.I. Description of a juvenile megamouth shark, Megachasma pelagios, caught off Brazil. Environ. Biol. Fishes 2000, 59, 117–123. [Google Scholar] [CrossRef]
- Tomita, T.; Tanaka, S.; Sato, K.; Nakaya, K. Pectoral Fin of the Megamouth Shark: Skeletal and Muscular Systems, Skin Histology, and Functional Morphology. PLoS ONE 2014, 9, e86205. [Google Scholar] [CrossRef]
- Berra, T.M.; Hutchins, J.B. A specimen of megamouth shark, Megachasma pelagios (Megachasmidae) from Western Australia. Rec. West. Aust. Mus. 1990, 14, 651–656. [Google Scholar]
- Yano, K.; Yabumoto, Y.; Tanaka, S.; Tsukada, O.; Furuta, M. Capture of a mature female megamouth shark, Megachasma pelagios, from Mie, Japan. In Proceedings of the 5th Indo-Pacific Conference, Nouméa, New Caledonia, 3–8 November 1997; pp. 335–349. [Google Scholar]
- White, W.T.; Fahmi, M.A.; Sumadhiharga, K. A juvenile megamouth shark Megachasma pelagios (Lamniformes: Megachasmidae) from Northern Sumatra, Indonesia. Raffles Bull. Zool. 2004, 52, 603–607. [Google Scholar]
- Lee, P.F.; Shao, K.T. Two new records of Lamniform shark from the waters adjacent to Taiwan. J. Fish. Soc. Taiwan 2009, 36, 303–311. [Google Scholar]
- Castillo-Géniz, J.L.; Ocampo-Torres, A.I.; Shimada, K.; Rigsby, C.K.; Nicholas, A.C. Juvenile megamouth shark, Megachasma pelagios, caught off the Pacific coast of Mexico, and its significance to chondrichthyan diversity in Mexico. Cienc. Mar. 2012, 38, 467–474. [Google Scholar] [CrossRef]
- Sawamoto, S.; Matsumoto, R. Stomach contents of a megamouth shark Megachasma pelagios from the Kuroshio Extension: Evidence for feeding on a euphausiid swarm. Plankton Benthos Res. 2012, 7, 203–206. [Google Scholar] [CrossRef] [Green Version]
- Currie, P.J. Allometric growth in tyrannosaurids (Dinosauria: Theropoda) from the Upper Cretaceous of North America and Asia. Can. J. Earth Sci. 2003, 40, 651–665. [Google Scholar] [CrossRef]
- Campione, N.E.; Evans, D.C. A universal scaling relationship between body mass and proximal limb bone dimensions in quadrupedal terrestrial tetrapods. BMC Biol. 2012, 10, 60. [Google Scholar] [CrossRef] [Green Version]
- Engelman, R. Giant, swimming mouths: Oral dimensions of extant sharks do not accurately predict body size in Dunkleosteus terrelli (Placodermi: Arthrodira). PeerJ 2023, 11, e15131. [Google Scholar] [CrossRef]
- Brown, C.M.; Vavrek, M.J. Small sample sizes in the study of ontogenetic allometry; implications for palaeobiology. PeerJ 2015, 3, e818. [Google Scholar] [CrossRef] [Green Version]
- Houck, M.A.; Gauthier, J.A.; Strauss, R.E. Allometric Scaling in the Earliest Fossil Bird, Archaeopteryx lithographica. Science 1990, 247, 195–198. [Google Scholar] [CrossRef]
- Mallon, J.C.; Evans, D.C.; Zhang, Y.; Xing, H. Rare juvenile material constrains estimation of skeletal allometry in Gryposaurus notabilis (Dinosauria: Hadrosauridae). Anat. Rec. 2022, 306, 1–23. [Google Scholar] [CrossRef]
- Yun, C.-G.; Peters, G.F.; Currie, P.J. Allometric growth in the frontals of the Mongolian theropod dinosaur Tarbosaurus bataar. Acta Palaeontol. Pol. 2022, 67, 601–615. [Google Scholar] [CrossRef]
- Kim, S.H.; Shimada, K.; Rigsby, C.K. Anatomy and evolution of heterocercal tail in lamniform sharks. Anat. Rec. 2013, 296, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.P.; Motta, P.J. Patterns of growth and the effects of scale on the feeding kinematics of the nurse shark (Ginglymostoma cirratum). J. Zool. 2002, 256, 449–462. [Google Scholar] [CrossRef]
- Speed, C.W.; Field, I.C.; Meekan, M.G.; Bradshaw, C.J.A. Complexities of coastal shark movements and their implications for management. Mar. Ecol. Prog. Ser. 2010, 408, 275–293. [Google Scholar] [CrossRef] [Green Version]
- Stone, N.R.; Shimada, K. Skeletal Anatomy of the Bigeye Sand Tiger Shark, Odontaspis noronhai (Lamniformes: Odontaspididae), and Its Implications for Lamniform Phylogeny, Taxonomy, and Conservation Biology. Copeia 2019, 107, 632–652. [Google Scholar] [CrossRef]
- Vella, N.; Vella, A. The complete mitogenome of the Critically Endangered smalltooth sand tiger shark, Odontaspis ferox (Lamniformes: Odontaspididae). Mitochondrial DNA B Resour. 2020, 5, 3319–3322. [Google Scholar] [CrossRef] [PubMed]
- Sternes, P.C.; Wood, J.J.; Shimada, K. Body forms of extant lamniform sharks (Elasmobranchii: Lamniformes), and comments on the morphology of the extinct megatooth shark, Otodus megalodon, and the evolution of lamniform thermophysiology. Hist. Biol. 2022, 35, 1–13. [Google Scholar] [CrossRef]
- Garrick, J.A.F. Additional information on the morphology of an embryo whale shark. Proc. U. S. Natl. Mus. 1964, 115, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Lisney, T.J.; Collin, S.P. Relative eye size in elasmobranchs. Brain Behav. Evol. 2007, 69, 266–279. [Google Scholar] [CrossRef]
- Compagno, L.J.V. Relationships of the megamouth shark, Megachasma pelagios (Lamniformes: Megachasmidae), with comments on its feeding habits. In Elasmobranchs as Living Resources: Advances in the Biology, Ecology, Systematics, and the Status of the Fisheries; Pratt, H.L., Gruber, S.H., Taniuchi, T., Eds.; NOAA Technical Report NMFS; NMFS: Springfield, VA, USA, 1990; pp. 357–379. [Google Scholar]
- Nakaya, K.; Matsumoto, R.; Suda, K. Feeding strategy of the megamouth shark Megachasma pelagios (Lamniformes: Megachasmidae). J. Fish Biol. 2008, 73, 17–34. [Google Scholar] [CrossRef]
- Tomita, T.; Sato, K.; Suda, K.; Kawauchi, J.; Nakaya, K. Feeding of the megamouth shark (Pisces: Lamniformes: Megachasmidae) predicted by its hyoid arch: A biomechanical approach. J. Morphol. 2011, 272, 513–524. [Google Scholar] [CrossRef]
- Stiefel, K.M. Evolutionary trends in large pelagic filter-feeders. Hist. Biol. 2020, 33, 1477–1488. [Google Scholar] [CrossRef]
- Setyawan, E.; Stevenson, B.C.; Izuan, M.; Constantine, R.; Erdmann, M.V. How Big Is That Manta Ray? A Novel and Non-Invasive Method for Measuring Reef Manta Rays Using Small Drones. Drones 2022, 6, 63. [Google Scholar] [CrossRef]
- Wilga, C.D.; Motta, P.J.; Sanford, C.P. Evolution and ecology of feeding in elasmobranchs. Integr. Comp. Biol. 2007, 47, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Nakaya, K.; Tomita, T.; Suda, K.; Sato, K.; Ogimoto, K.; Chappell, A.; Sato, T.; Takano, K.; Yuki, Y. Slingshot feeding of the goblin shark Mitsukurina owstoni (Pisces: Lamniformes: Mitsukurinidae). Sci. Rep. 2016, 6, 27786. [Google Scholar] [CrossRef]
- Sims, D.W. Filter-feeding and cruising swimming speeds of basking sharks compared with optimal models: They filter-feed slower than predicted for their size. J. Exp. Mar. Biol. Ecol. 2000, 249, 65–76. [Google Scholar] [CrossRef]
- Sims, D.W. Can threshold foraging responses of basking sharks be used to estimate their metabolic rate? Mar. Ecol. Prog. Ser. 2000, 200, 289–296. [Google Scholar] [CrossRef] [Green Version]
- Sims, D.W. Sieving a living: A review of the biology, ecology and conservation status of the plankton-feeding basking shark Cetorhinus maximus. Adv. Mar. Biol. 2008, 54, 171–220. [Google Scholar]
- Ferry, L.A.; Paig-Tran, E.M.; Gibb, A.C. Suction, Ram, and Biting: Deviations and Limitations to the Capture of Aquatic Prey. Integr. Comp. Biol. 2015, 55, 97–109. [Google Scholar] [CrossRef] [Green Version]
- Wolfson, F.H. Records of seven juveniles of the whale shark, Rhiniodon typus. J. Fish Biol. 1983, 22, 647–655. [Google Scholar] [CrossRef]
- Wainwright, P.; Carroll, A.M.; Collar, D.C.; Day, S.W.; Higham, T.E.; Holzman, R.A. Suction feeding mechanics, performance, and diversity in fishes. Integr. Comp. Biol. 2007, 47, 96–106. [Google Scholar] [CrossRef] [Green Version]
- Richard, B.A.; Wainwright, P.C. Scaling the feeding mechanism of largemouth bass (Micropterus salmoides): Kinematics of prey capture. J. Exp. Biol. 1995, 198, 419–433. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, P.C.; Richard, B.A. Predicting patterns of prey use from morphology of fishes. Environ. Biol. Fishes 1995, 44, 97–113. [Google Scholar] [CrossRef]
- Yokogawa, K. Morphological variations in the largemouth bass Micropterus salmoides with particular emphasis on growth-related changes. Aquac. Sci. 2014, 62, 361–374. [Google Scholar]
- Pyenson, N.D.; Goldbogen, J.A.; Shadwick, R.E. Mandible allometry in extant and fossil Balaenopteridae (Cetacea: Mammalia): The largest vertebrate skeletal element and its role in rorqual lunge-feeding. Biol. J. Linn. Soc. 2013, 108, 586–599. [Google Scholar] [CrossRef] [Green Version]
- Kahane-Rapport, S.R.; Goldbogen, J.A. Allometric scaling of morphology and engulfment capacity in rorqual whales. J. Morphol. 2018, 279, 1256–1268. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.Y.; Papastamatiou, Y.P. Biologging and Biotelemetry: Tools for Understanding the Lives and Environments of Marine Animals. Annu. Rev. Anim. Biosci. 2023, 11, 247–267. [Google Scholar] [CrossRef]





| Specimen ID | BPBM 22730 | N/A | WAM P.29940-001 | N/A | MZB12906 | ASIZP0071582 | NMMBP8950 | SIO 07-53 | N/A |
|---|---|---|---|---|---|---|---|---|---|
| Ontogenetic stage | Adult | Juvenile | Adult | Adult | Juvenile | Adult | Adult | Juvenile | Subadult |
| Sex | Male | Male | Male | Female | Male | Female | Female | Female | Female |
| Body Length (mm) | 4460 | 1900 | 5150 | 5440 | 1767 | 4830 | 4870 | 2265 | 3667 |
| Location | Hawaii, USA | Brazil | Australia | Mikizaki, Japan | Sumatra, Indonesia | Hualien, Taiwan | Hualien, Taiwan | Mexico | Ibaraki, Japan |
| Source | [1] | [23] | [25] | [26] | [27] | [28] | [28] | [29] | [30] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, C.-g.; Watanabe, Y.Y. Allometric Growth of the Enigmatic Deep-Sea Megamouth Shark Megachasma pelagios Taylor, Compagno, and Struhsaker, 1983 (Lamniformes, Megachasmidae). Fishes 2023, 8, 300. https://doi.org/10.3390/fishes8060300
Yun C-g, Watanabe YY. Allometric Growth of the Enigmatic Deep-Sea Megamouth Shark Megachasma pelagios Taylor, Compagno, and Struhsaker, 1983 (Lamniformes, Megachasmidae). Fishes. 2023; 8(6):300. https://doi.org/10.3390/fishes8060300
Chicago/Turabian StyleYun, Chan-gyu, and Yuuki Y. Watanabe. 2023. "Allometric Growth of the Enigmatic Deep-Sea Megamouth Shark Megachasma pelagios Taylor, Compagno, and Struhsaker, 1983 (Lamniformes, Megachasmidae)" Fishes 8, no. 6: 300. https://doi.org/10.3390/fishes8060300